2. Department of Pediatrics, Regina General Hospital, Regina, SK, Canada;
3. Department of Community Health and Epidemiology, Royal University Hospital, University of Saskatchewan, Saskatoon, SK, Canada;
4. College of Medicine, University of Saskatchewan, Saskatoon, SK, Canada
2. Department of Pediatrics, Regina General Hospital, Regina, SK, Canada;
3. Department of Community Health and Epidemiology, Royal University Hospital, University of Saskatchewan, Saskatoon, SK, Canada;
4. College of Medicine, University of Saskatchewan, Saskatoon, SK, Canada
Respiratory syncytial virus (RSV) is the most common cause of respiratory tract infection in children under age 2 worldwide [1] ,with 90% of children infected by their second birthday [2] . Up to 40% of these children will have an acute lower respiratory tract infection (LRTI) [3] . In Canada,RSV is responsible for many of the 12 000 hospitalizations annually for bronchiolitis in children under the age of two [4] . RSV is still a leading viral cause of death in infants [5] . In the United States,RSV is responsible for 100-500 deaths each year,with most of them being in children under one year-of-age [6] . Nair et al [5] estimate that 66 000-199 000 children under age 5 died from RSV associated LRTIs worldwide in 2005. Health care spending for RSV related hospital admissions in Canada is $18 million (US) annually [7] .
Premature infants <35 weeks gestation and those with chronic lung disease or hemodynamically significant congenital heart disease are at the highest risk of morbidity and mortality from RSV [1,8] . Infants with chronic lung disease related to prematurity have high rates of hospitalization for LRTI,and have increased vulnerability to severe RSV disease [9] . High risk groups,including the above and those with other comorbid conditions such as neurologic impairment,are at higher risk of death during RSV hospitalization [10] . In Canada,infants in remote and northern communities and those of aboriginal descent are thought to be at increased risk for RSV [11] . Palivizumab,a humanized monoclonal RSV antibody, has been shown to be well tolerated and to decrease the risk of RSV-related hospitalizations in high risk children,including premature infants up to 35 weeks gestation at birth [12,13] .
There have been no studies reviewing the epidemiology of RSV infection and its burden to society in Canada within a defined population and over a specific time period. Further,there is very little information on those infants who contract RSV disease after prophylaxis. The purpose of this study was to describe the epidemiology of children hospitalized due to RSV in the province of Saskatchewan,with a population of one million,as well as determine the severity of RSV infection in those who required hospitalization despite palivizumab prophylaxis. Materials and methods Patients
The Canadian Paediatric Society (CPS) recommends palivizumab for children <24 months of age with chronic lung disease (CLD) of prematurity who require ongoing medical therapy within the six months preceding the RSV season,and children <24 months of age with hemodynamically significant heart disease. For preterm infants,the CPS recommended palivizumab to infants born at <32 weeks (rather than <35 weeks) gestation who are <6 months old at the start of the RSV season (includes those with or without CLD),and included children born at <36 weeks gestation who reside in northern or rural remote communities and Inuit infants if they are <6 months of age at the beginning of RSV season [8] . These CPS recommendations were implemented and received funding from Saskatchewan Health between 1999 and 2005.
This study included all children (from birth to 16 years and 11 months) hospitalized with known RSV infection for greater than 24 hours at the only two tertiary pediatric care centers in the province of Saskatchewan,Royal University Hospital (RUH) in Saskatoon,and Regina General Hospital (RGH) in Regina,during the study period (July 2002 to June 2005). These two tertiary pediatric centers serve the entire province,with a population of approximately 970 000 during the study period [14] . Patients were identified using virology records. For patients admitted at RUH,RSV was deemed to be present if there was a positive Direct Fluorescent Antibody (DFA,SimulFluor RSV/Flu A Kit,Millipore, Billerica,MA) result performed on a nasopharyngeal aspirate (NPA). Viral culture was only performed on DFA negative specimens in Saskatoon. For patients admitted at RGH,RSV was deemed to be present if there was a positive DFA (SimulFluor Respiratory Screen) and/or viral culture (HEp-2 cells) for RSV performed on nasopharyngeal swabs (nylon-flocked tips,microRheologics,Copan Diagnostics,Corona, CA) at the provincial Laboratory.
Once patients were identified,hospital records were retrospectively reviewed for the following demographic criteria: gender,ethnicity,and residence/ community. Past medical history was noted for: chronological age at admission,gestational age at birth,presence of underlying medical conditions,need for home oxygen prior to admission,and whether the patient has received palivizumab prophylaxis prior to admission. The course in hospital was reviewed for: mode of transportation to hospital,type and severity of RSV infection,total duration of hospital admission, need for and duration of pediatric intensive care, need for and duration of intubation and mechanical ventilation,secondary bacterial infections,and outcomes,including RSV related deaths.
As there were no available case definitions measuring severity of RSV disease requiring hospitalization,we developed our own. Mild illness was defined as no change in oxygen requirement, hospitalized for <48 hours and no secondary complications. Moderate illness included those who were hospitalized for 2-6 days,had increased oxygen requirement for at least 12-24 hours (based on an oxygen saturation less than 92% in room air),and no more than 5% dehydration (still able to feed,but may have some vomiting or diarrhea). Severe illness was defined as those with respiratory distress requiring PICU admission,and/or intubation and ventilation, complications such as pneumothorax,secondary bacterial infection (pneumonia,pleural effusion, sepsis) requiring antibiotic treatment,a total hospital duration (including PICU) of 7 days or more,or death. Secondary bacterial infection was defined as having a positive blood,NPA,or sputum culture.
Eligibility criteria for palivizumab prophylaxis in Saskatchewan during the study period were consistent with the recommendations of the CPS,first published in 1999 [15,16] . From 2004 on,the CPS further recommended that children < 2 years of age with hemodynamically significant cyanotic or acyanotic congenital heart disease (requiring corrective surgery or on cardiac medication for hemodynamic considerations) also receive palivizumab prophylaxis.
The study was approved by the Biomedical Ethics Research Board for the University of Saskatchewan. Statistical analyses
Descriptive statistics (i.e. proportions,medians) were obtained using SPSS 17.0. Since patients admitted at RUH and RGH were similar,we combined data from the two centers for analysis. The MannWhitney test was applied for comparing medians and for categorical data,the Pearson Chi-Square statistic was used to test associations and Fisher's exact test if expected cell frequencies were less than five. Results
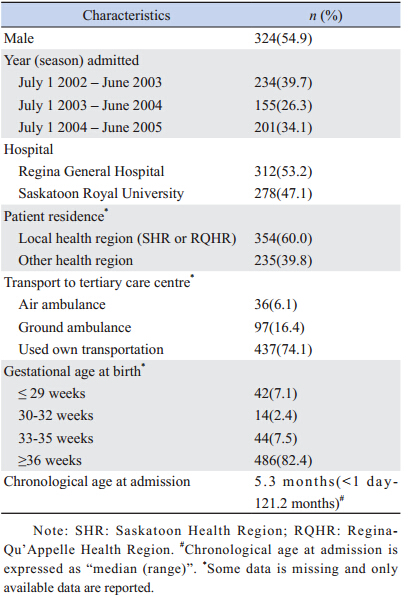
A total of 590 children (324 males) accounted for 602 hospital admissions for RSV infection over the 3 years,with twelve children having two hospitalizations each. The characteristics of these patients are summarized in Table 1. Three-hundred and twelve children (53.2%) were hospitalized at RGH and 278 children (47.1%) at RUH. Thirtysix (6.1%) patients required transportation by air ambulance to the tertiary care center,while 97 (16.4%) required ground ambulance transport. The median chronological age at admission was 5.3 months (range <1 day-121.2 months). The gestational age at birth was ≥ 36 weeks in 82.4%,33-35 weeks in 7.5%,and ≤ 32 weeks in 9.5% of patients.
| Table 1 Demographics and patterns of referral for 590 subjects |
The distribution of cases throughout the months based on date of RSV positive test can be seen in Figure 1. RSV cases followed a seasonal pattern,with the majority of cases occurring in the winter months. In 2004,the RSV season was slightly delayed,with more cases occurring from March to June.
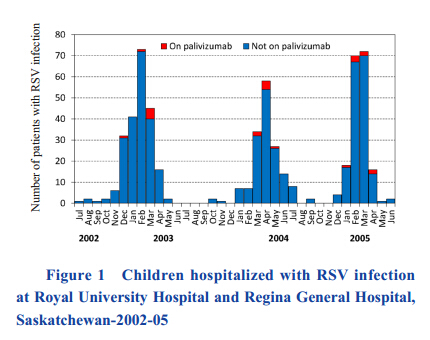 |
Figure 1 Children hospitalized with RSV infection at Royal University Hospital and Regina General Hospital, Saskatchewan-2002-05 |
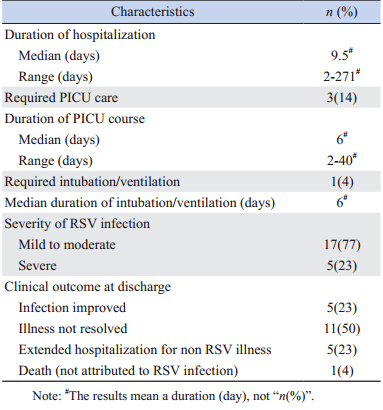
Clinical presentation at time of hospitalization included bronchiolitis (70.2%),pneumonia (38.1%), upper respiratory infection (URI) (17.3%) and croup (0.5%) (Table 2,patients may have more than one clinical presentation). Infections were classified as mild to moderate in 478 patients (81.0%),while 110 (18.6%) were considered severe. The median duration of hospitalization was 4 days (range 2-367 days). The vast majority of patients,548 (92.9%),required general pediatric ward stay only,with 39 (6.6%) requiring PICU care. Nineteen patients (3.2%) required mechanical ventilation. Twenty-five percent of patients had a secondary bacterial infection. No deaths were attributed to RSV infection. Although the majority of the patients had infections that had resolved or were improving from the infection at hospital discharge,36 patients (6.1%) required prolonged hospitalization,all for non-RSV related reasons.
| Table 2 Clinical data and hospital care for 590 subjects |
| Table 3 Demographic and clinical characteristics for 22 patients receiving palivizumab prophylaxis |
| Table 4 Hospitalization,severity and outcomes for 22 patients receiving alivizumab prophylaxis |
Of those 39 patients requiring PICU admission, 22 (56%) were male. The median duration of PICU stay was 5 days (range 0-92 days). Fourteen PICU patients (36%) had a secondary bacterial infection,the most common being H. influenza(36%),followed by S. pneumoniae(21%). Compared to patients admitted only to the general pediatric ward,those admitted to PICU were more likely to: be younger at admission (P<0.01),have an underlying lung disease (P<0.01) or congenital heart disease (P=0.01),have two or more underlying illnesses (P<0.01),and have other children residing in the household (P=0.02).
Nineteen of the children admitted to PICU (49%) were intubated and mechanically ventilated. The median length of intubation was 6 days (range 3-34 days). Pneumonia (P=0.02) and secondary bacterial infections (P=0.01) were more prevalent among ventilated patients than in those not ventilated,though no other statistically significant differences emerged. Patients who had received palivizumab
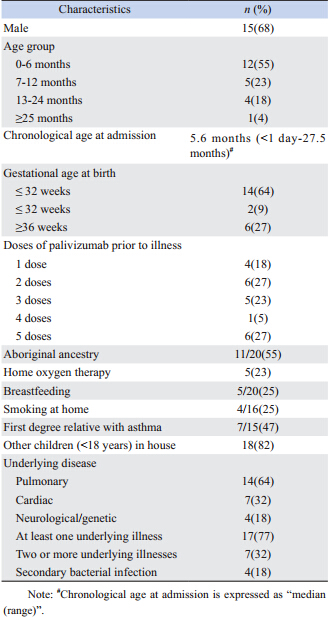
Twenty-two patients had received palivizumab prophylaxis. Demographic,clinical and hospitalization data for these patients are shown in Tables 3 and 4. Only 12 patients (55%) had received >3 doses of palivizumab before being hospitalized.
Overall,17 (77%) of these patients had one or more chronic medical illnesses. These included underlying lung,heart,and neurological/genetic conditions in 14 (64%),7 (32%),and 4 (18%) patients,respectively. The median chronological age at admission was 5.6 months (range 0-27.5 months) and median duration of hospitalization was 9.5 days (range 2-271 days). Sixty-four percent (14/22) were <32 weeks gestation at birth. RSV illness was considered severe in 23% (5/22),with 14% (3/22) requiring PICU care for a median of 6 days (range 2-40 days); one child was intubated for 6 days. One death occurred, although not attributed to RSV.
Figure 1 also shows the distribution of RSV positive cases in those who had received palivizumab prophylaxis. These cases were distributed throughout the RSV season,with no cases in the summer months. Discussion
This is the first study to document the epidemiology of patients presenting with RSV to the only two tertiary care centers in Saskatchewan, Canada,and gives insight into overall burden of the disease. Typically all infants with moderate to severe LRTI are referred to these two centers therefore this is an accurate reflection of moderate to severe RSV disease in a defined population of one million.
These patients are more likely to be male,<1 year of age,and be >35 weeks gestation at birth, consistent with previous studies [8,17] . The most common presentation was bronchiolitis,most children had mild to moderate illness,and there was no mortality attributed to RSV infection. This is interesting to note,because the majority were late preterm and term infants,suggesting a shift of burden of disease from infants born preterm to infants born at later gestation.
Risk factors less consistently linked with RSV hospitalization include birth between October and December,birth weight less than 2 500 g,male sex, age less than 12 months,low socioeconomic status, daycare attendance,school-aged siblings,exposure to second hand smoke,congenital airway anomalies,and severe neuromuscular disease [8,9] . Recommendations were made to provide palivizumab prophylaxis to those born at 33-35 weeks gestation using a risk factor scoring system based on later studies [18,19,20] ,but were only implemented in Saskatchewan after 2005. The study period was chosen to reflect the epidemiology of RSV disease before eligibility for palivizumab prophylaxis was widened to include children born at 33-35 weeks gestation.
Prior to commencement of this chart review, definitions of severity of illness were not available in the literature. The authors developed these definitions based on clinical experience. Although the definitions intuitively make sense,they have not been validated.
Twenty-five percent of 590 patients in this study had secondary bacterial infections,which is higher than that found in previous studies [21,22] . However, one of our two centers documented all positive blood cultures,which may have included contaminants, artificially elevating the secondary bacterial infection rate. For organisms isolated from sputum or endotracheal respiratory specimens,they may also reflect colonization,rather than true lower respiratory tract infections. Our data does not include timing of the diagnosis of secondary bacterial infection with diagnosis of the RSV infection,so we cannot determine which came first. One study in Japan found rates of respiratory bacterial co-infection of 43.6% based on sputum bacterial culture,though the clinical significance is unclear as not all patients required treatment with antibiotics [23] .
Children admitted to PICU tended to be of younger chronological age,with a median age of 2.96 months. This is similar to other studies,specifically linking a chronological age of <6 weeks with ICU admission [24,25] . In this study,most patients admitted to the PICU (84%) were >35 weeks gestational age at birth. This is in keeping with other studies,and suggests that palivizumab prophylaxis is unlikely to have a significant effect on the burden of RSV disease in the intensive care setting [9,17,26,27] .
Similar to other studies,children requiring PICU were more likely to have other children in the house and were more likely to have an underlying illness [25,28] . Those with underlying illness were also more likely to be intubated and mechanically ventilated,again a known risk factor [29] . Secondary bacterial infections were more common in those admitted to PICU,particularly in those who were intubated and mechanically ventilated,consistent with other studies where rates of bacterial co-infection in patients with severe RSV disease have been reported of 20%-40% [30,31] . Most in this study were respiratory pathogens. The data does not allow for a differentiation between pneumonia and secondary bacterial infection associated solely with RSV and those infections associated with intensive care itself (i.e. ventilator associated pneumonia,bacteremia secondary to central line).
Some children who had received palivizumab prophylaxis still required hospitalization for RSV. Most were classified as mild to moderate illness,and only 14% of these children required PICU admission. This is consistent with previous studies,including a recent Cochrane review,which showed palivizumab to be effective at decreasing hospitalization and severity of RSV infection [3,32] ,as well as a comparison of two Canadian centers that showed a reduction of hospitalization of high risk infants for RSV infection from 7.3% to 3.0% in the center where prophylaxis was offered [4] . In the center where palivizumab was not offered,there was no reduction in the rate of hospitalization.
In this study,patients who received palivizumab had a younger gestational age at birth,not surprising as this is one of the major criteria to qualify for RSV prophylaxis. A large number had underlying pulmonary disease,again likely related to criteria for receiving palivizumab. CARESS (the Canadian Registry of Palivizumab) found that in Canada, patients were more likely to be hospitalized with RSV post-palivizumab prophylaxis if there was atopy in the immediate family,mother smoked in pregnancy,there were smokers in the home environment,2 smokers in the household,siblings,higher birth weight,and if the infant had received fewer palivizumab injections [33] . This study did not look at most of these factors, though having siblings in the house was common. The distribution of RSV positive cases in those who had received palivizumab was seasonal,similar to the rest of the cases of RSV. Cases did not occur earlier in the season,suggesting that children who had received multiple doses still required hospitalization,though the numbers in this group are small so it is difficult to draw conclusions from this.
Saskatchewan has a proximately 15% aboriginal population,and in this study,55% of children receiving palivizumab were of aboriginal descent, but we are unable to make comparisons with the other children included in the study as this piece of information was often missing from the data available in the chart.
Limitations of this study included the retrospective nature,and the fact that RSV hospitalization numbers do not reflect the population incidence of RSV. We cannot draw conclusions about the general population rates and demographic criteria of patients with RSV who are seen as outpatients,or in primary or secondary care centers. There may also have been children admitted to these tertiary care centers with bronchiolitis who were not tested,and therefore not included in the study. The standard practice in these two hospitals at the time of the study would suggest that most patients were tested,as would have occurred in all patients in the PICU,but some patients with mild to moderate illness may not have been. The two hospitals used different testing methods for RSV during the time of the study (RUHviral culture only done if DFA negative,RGH-either viral culture or DFA),which may also have affected the results. These results are not generalizable to all patients admitted with RSV infection,as the study only included tertiary care sites,and not those admitted to primary or secondary sites. The numbers of patients requiring PICU care and those who had received palivizumab prophylaxis were small,making it difficult to draw any conclusions. We also do not have information available regarding the total number of children who received palivizumab prophylaxis, so cannot determine the percentage of children with palivizumab prophylaxis failure. It is possible that some children who received palivizumab prophylaxis were admitted to primary or secondary care sites, and so their admissions would not be included in this study. It appears that a disproportionate number of aboriginal infants are infected with RSV,but the lack of data surrounding the ethnic backgrounds of patients other than in the palivizumab group makes it difficult to interpret this. Further larger studies are needed to review the effects of ethnicity on RSV infection, as well as further characterize the demographic characteristics of patients requiring intensive care, and those who are hospitalized with RSV despite palivizumab prophylaxis.
| [1] | Paes BA, Mitchell I, Banerji A, et al. A decade of respiratorysyncytial virus epidemiology and pophylaxis: translatingevidence into everyday prophylaxis[J/OL]. Can Respir J, 2011,18(2): e10-e19. |
| [2] | Greenough A, Cox S, Alexander J, et al. Health care utilizationof infants with chronic lung disease, related to hospitalizationfor RSV infection[J]. Arch Dis Child, 2001, 85(6): 463-468. |
| [3] | Meissner HC. Selected populations at increased risk fromrespiratory syncytial virus infection[J]. Pediatr Infect Dis J,2003, 22(2 Suppl): S40-S44. |
| [4] | Mitchell A, Tough S, Gillis L, et al. Beyond randomizedcontrolled trials: a “real life” experience of respiratorysyncytial virus infection prevention in infancy with and withoutpalivizumab[J]. Pediatr Pulmonol, 2006, 41(12): 1167-1174. |
| [5] | Nair H, Nokes DJ, Gessner BD, et al. Global burden of acutelower respiratory infection due to respiratory syncytial virus inyoung children: a systematic reiew and meta-analysis[J]. Lancet,2010, 375(9725): 1545-1555. |
| [6] | Shay DK, Holman RC, Roosevelt GE, et al. Bronchiolitisassociatedmortality and estimates of respiratory syncytial virusassociateddeaths among US children, 1979-1997[J]. J InfectDis, 2001, 183(1): 16-22 |
| [7] | Resch B, Sommer C, Nuitjen M, et al. Cost-effectiveness ofpalivizumab for respiratory syncytial virus infection in high riskchildren, based on long-term epidemiologic data from Austria[J/OL]. Pediatr Infect Dis J, 2012, 31 (1): e1-e8. |
| [8] | Canadian Pediatric Society Infectious Diseases andImmunization Committee. Prevention of respiratory syncytialvirus infection[J]. Pediatr Child Health, 2009, 14(8): 521-526. |
| [9] | Langley GF, Anderson LJ. Epidemiology and prevention ofrespiratory syncytial virus infections among infants and youngchildren[J]. Pediatr Infect Dis J, 2011, 30(6): 510-517. |
| [10] | Welliver RC Sr, Checchia PA, Bauman JH, et al. Fatality rates inpublished reports of RSV hospitalizations among high-risk andotherwise healthy children[J]. Curr Med Res Opin, 2010, 26(9):2175-2181. |
| [11] | Banerji A, Greenberg D, White LF, et al. Risk factors associatedwith hospitalization due to lower respiratory tract infections inCanadian Inuit children: a case control study[J]. Pediatr InfectDis J, 2009, 28(8): 697-701. |
| [12] | Andabaka T, Nickerson JW, Rojas-Reyes MX, et al. Monoclonalantibody for reducing the risk of respiratory syncytial virusinfection in children (review) [J]. Cochrane Database ofSystematic Reviews, 2013, (4), art. no: CD006602. |
| [13] | Turti TV, Baibarina EN, Degtiareva EA, et al. A prospective,open-label, non-comparative study of palivizumab prophylaxisin children at high risk of serious respiratory syncytial virusdisease in the Russian Federation[J]. BMC Res Notes, 2012, 5:484-490. |
| [14] | Statistics Canada. 2007. Saskatchewan Landing No. 167;Saskatchewan (Code4708038) (table). 2006 CommunityProfiles. 2006 Census. Statistics Canada Catalogue no. 92-591-XWE. Ottawa [DB/OL]. Released March 13, 2007. http://www12.statcan.ca/census-recensement/2006/dp-pd/prof/92-591/index.cfm?Lang=E. |
| [15] | Tan B; Canadian Paediatric Society, Infectious Diseases andImmunization Committee. Palivizumab and respiratory syncytialvirus globulin intravenous for the prophylaxis of respiratorysyncytial virus infection in high risk infants[J]. Paediatr ChildHealth, 1999, 4 (7): 474-480. |
| [16] | Langley JM; Canadian Paediatric Society, Infectious Diseasesand Immunization Committee. Use of palivizumab in childrenwith congenital heart disease[J]. Paediatr Child Health, 2003,8(10): 631-633. |
| [17] | Hall CB, Weinberg GA, Iwane, MK et al. The burden ofrespiratory syncytial virus in young children[J]. N Engl J Med2009, 360 (6): 588-598. |
| [18] | Meissner HC. Commentary: the unresolved issue of risk factorsfor hospitalization of infants with respiratory syncytial virusinfection born after 33-35 weeks gestation[J]. Pediatr Infect DisJ, 2004, 23 (9): 821-823. |
| [19] | Law B, Langley J, Allen U, et al. The pediatric investigatorscollaborative network on infections in Canada study ofpredictors of hospitalization for respiratory syncytial virusinfection for infants born at 33 through 35 weeks gestation[J].Pediatr Infect Dis J, 2004, 23 (9): 806-814. |
| [20] | Figueras-Aloy J, Carbonell-Estrany X, Quero J. Case-controlstudy of the risk factors linked to respiratory syncytial virusinfection requiring hospitalization in premature infants born atgestational age of 33-35 weeks in Spain[J]. Pediatr Infect Dis J,2004, 23 (9): 815-820. |
| [21] | Hall CB, Powell KR, Schnabel KC, et al. Risk of secondarybacterial infection in infants hospitalized with respiratorysyncytial virus infection[J]. J Pediatr, 1988, 113 (2): 266-271. |
| [22] | Kupperman N, Bank DE, Walton EA, et al. Risks for bacteremiaand urinary tract infection in young febrile children withbronchiolitis[J]. Arch Pediatr Adolesc Med, 1997, 151(12):1207-1214. |
| [23] | Hishiki H, Ishiwada N, Fukasawa C. Incidence of bacterialcoinfection with respiratory syncytial virus bronchopulmonaryinfection in pediatric inpatients[J/OL]. J Infect Chemother, 2011,17(1): 87-90. |
| [24] | Hon KL, Leung TF, Cheng WY, et al. Respiratory syncytialvirus morbidity, premorbid factors, seasonality, and implicationsfor prophylaxis[J]. J Crit Care 2012, 27 (5): 464-468. |
| [25] | Crowcroft NS, Zambon M, Harrison TG, et al. Respiratorysyncytial virus infection in infants admitted to paediatricintensive care units in London and in their families[J]. Eur JPediatr, 2008, 167(4): 395-399. |
| [26] | Berger TM, Aebi C, Duppenthaler A, et al. Prospectivepopulation-based study of RSV-related intermediate care andintensive care unit admissions in Switzerland over a 4-yearperiod (2001-2005)[J]. Infection, 2009, 37(2): 109-116. |
| [27] | Prais D, Danino D, Schonfeld T, et al. Impact of palivizumabon admission to the ICU for respiratory syncytial virusbronchiolitis: a national survey[J]. Chest, 2005, 128(4): 2765-2771. |
| [28] | Sommer C, Resch B, Simoes EAF. Risk factors for severerespiratory syncytial virus lower respiratory infection[J]. OpenMicrobiol J, 2011, 5(Suppl 2-M4): 144-154. |
| [29] | Purcell K, Fergie J. Driscoll Children's Hospital respiratorysyncytial virus database: risk factors, treatment, and hospitalcourse in 3308 infants and young children 1991 to 2002[J].Pediatr Infect Dis J, 2004, 23(5): 418-423. |
| [30] | Levin D, Tribuzio M, Green-Wrzesinski T, et al. Empiricantibiotics are justified for infants wiwth respiratory syncytialvirus lower respiratory tract infection presenting with respiratoryfailure: a prospective study and evidence review[J]. Pediatr CritCare Med, 2010, 11(3): 390-395. |
| [31] | Thorburn K, Harigopal S, Reddy V, et al. High incidenceof pulmonary bacterial co-infection in children with severerespiratory syncytial virus (RSV) bronchiolitis[J]. Thorax, 2006,61(7): 611-615. |
| [32] | The Impact-RSV Study Group. Palivizumab, a humanizedrespiratory syncytial virus monoclonal antibody, reduceshospitalization from respiratory syncytial virus infection in highrisk infants[J]. Pediatrics, 1998, 102(3): 531-537. |
| [33] | Mitchell I, Paes BA, Li A, et al. CARESS: The Canadianregistry of palivizumab[J]. Pediatr Infect Dis J, 2011, 30(8):651-655. |
 2014, Vol. 16
2014, Vol. 16