2. Department of Pediatrics/Neonatology, Maastricht University Medical Center, The Netherlands
Adequate pulmonary function is a prerequisite for survival of preterm infants. Preterm birth is highly associated with the respiratory distress syndrome (RDS),caused by structural and functional immaturity of the newborn lung. Beside simple structural immaturity,the preterm lung is in addition susceptible to injury resulting from different prenatal conditions like intra-uterine growth restriction or oligohydramnios,genetic disposition,transition at birth and postnatal procedures and insults (Figure 1).However,these early alterations may also interfere with lung development,and therefore exert lasting effects on pulmonary plasticity and integrity,finally resulting in structural and functional impairment.
Mechanisms leading to acute and chronic lung injury have been elucidated experimentally in various animal models. These experiments have helped to understand clinical findings and to develop therapeutic interventions. Moreover,they allowed investigating complex interaction between fetal,perinatal and postnatal factors modulating lung injury. In this review we will focus on experimental animal models in the field of neonatal pulmonary biology for lung injury mediated by and related to inflammatory changes in the lung.
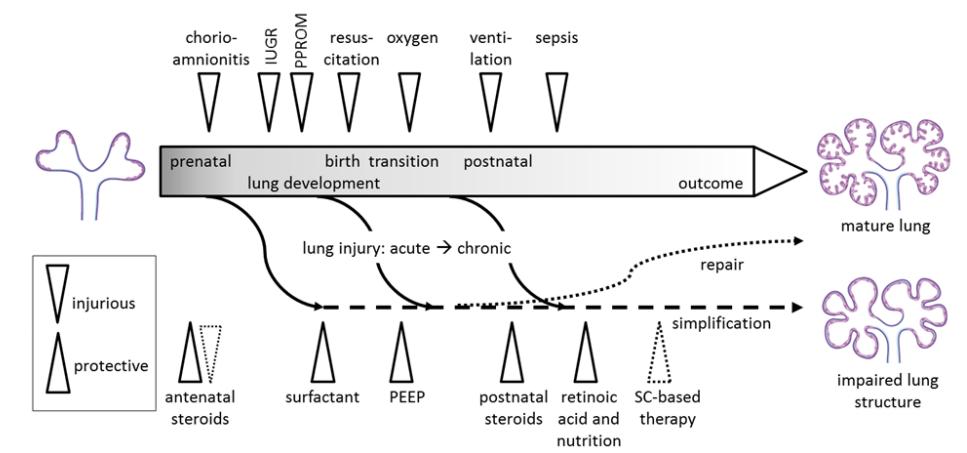 |
Figure 1 Mechanisms of lung injury and protection from lung injury during prenatal and postnatal development IUGR: intrauterine growth restriction; PPROM: preterm prelabor rupture of membranes; SC: stem cells; PEEP: positive end exspiratory pressure. |
Chorioamnionitis,defined as inflammation of the membranes with or without involvement of the fetus,is a very common finding in infants born prematurely [1] . A modulating role of intrauterine exposure to inflammation on lung immaturity has been recognized since many years [2] . Fetal signs of chorioamnionitis have been shown to have an additional effect on RDS reduction,suggesting a “dose”-dependent response [3] . Despite a reduction in acute respiratory problems,several early human studies have shown chorioamnionitis to be associated with a paradoxical increase in chronic lung disease of prematurity,bronchopulmonary dysplasia (BPD) [4, 5] . However,more recent studies show considerable heterogeneity regarding this association [4] .
Part of the inconsistency among studies may be explained by the imprecise diagnosis of both RDS and BPD [6, 7] . Moreover,several confounders affect the relationship between chorioamnionitis and BPD. The most obvious one is gestational age,which is inversely correlated with the incidence of both entities. Thus, chorioamnionitis-exposed infants may be at higher risk for BPD merely because of their lower gestational age. However,recent epidemiological studies give an intriguing additional explanation,and support a “second hit” hypothesis [8] . In these particular studies, chorioamnionitis was associated with a decreased rather than increased risk for BPD in preterm infants [5, 8] . In one study,the effect was reversed when adjusted for mechanical ventilation [5] . If mechanical ventilation exceeded one week in chorioamnionitisexposed infants,the risk for BPD was increased [8] . In addition,postnatal sepsis also highly increased BPD risk in infants with chorioamnionitis in both studies [5, 8] .
Mechanisms of chorioamnionitis-induced lung injury were studied in different animal models. These provided growing insight into the interplay between intrauterine inflammatory stimuli and fetal lung injury, fetal pulmonary and systemic inflammatory response, and repair,and partly revealed mechanisms altering lung structure and lung function on a molecular and cellular level [9] .
As inflammatory mediator,interleukin (IL)-1α was first shown to accelerate surfactant protein synthesis and lung stability in a model utilizing fetal rabbits [10] . In a sheep model,intraamniotic IL-1α initiated pulmonary inflammation [11] and induced a maturation response,depicted by increased alveolar saturated phosphatidylcholines and improved lung function [11] . These maturational effects were also observed when IL-1α was administered directly into the trachea of fetal lambs,suggesting a direct reaction of lung epithelial cells [12] . However,effects of IL-1α on surfactant protein expression depended on gestational age in a rabbit lung explant model [13] .
Furthermore,IL-1 mediated lung inflammation and injury was induced by intraamniotic administration of E.coli-derived Lipopolysaccharide (LPS),while blocking of IL-1 signaling with a receptor antagonist decreased LPS-induced lung inflammation and maturation in sheep [14] . The increase of inflammatory cells in alveolar lavage and fetal lung tissue depended on the dose of endotoxins given [15] .
Concomitantly,intraamniotic LPS-exposure led to an increase in lung cytokine mRNA expression,shown for IL-1β,IL-6,IL-8 and tumor necrosis factor (TNF) α [16] .
In order to mimic clinical situation,the sheep model was further developed by utilizing intraamniotic Ureaplasma [17] . Chorioamnionitis is associated with polymicrobial colonization of the amniotic fluid [18] , with Ureaplasma the most common isolate. In previous clinical studies,Ureaplasma colonization of the preterm lung had been linked to BPD [19] . In a sheep model,acute Ureaplasma infection moderately increased lung monocytes and neutrophils [20] .
In contrast to LPS-induced chorioamnionitis, maturational effects on surfactant protein expression were not seen when in this model sheep were only exposed to Ureaplasma for several days. However, Ureaplasma is also capable of maintaining silent chronic infection in the amnion. Experimental data revealed stable colonization of fetal sheep lungs with Ureaplasma which was administered at early and midgestation [21, 22] . At term,interleukin-8 levels in the fetal lung had doubled in the Ureaplasma-colonized animals,and surfactant levels in bronchoalveolar lavage fluid had increased [22] .
In summary,these findings indicate that etiology,time point and duration of infection have an impact on pulmonary inflammation and induction of maturation. Additionally,different inflammatory agonists led to different subsequent phenotypes of lung injury. While intraamniotic IL-1 did not cause morphometric changes in fetal lamb lungs,depicted by alveolar surface and alveolar wall thickness [12] , intraamniotic LPS exposure reduced alveolar surface area and caused thinning of the alveolar wall when administered in early gestation in fetal lambs [23] . In a sheep model of acute intraamniotic Ureaplasma infection,morphological changes of pulmonary extracellular matrix protein expression became evident,comparable to those changes found in BPD patients [20] . Early Ureaplasma infection resulted in higher lung gas volumes and higher surfactant lipid contents of lungs of prematurely delivered lambs [21] , whereas lung morphometry as well as elastin and collagen density remained unaltered [21] . Further experiments regarding Ureaplasma-induced lung alterations are necessary to elucidate their role in the development of BPD [17] .
However,to a certain extend the fetus seems to be capable of attenuating the inflammatory response [24, 25] . In fetal sheep receiving LPS for 28 days during mid-gestation,signs of mild persistent inflammation were present in the fetal lung,and surfactant saturated phosphatidylcholine pool sizes were increased [26] . When animals were delivered preterm,alveolar numbers were decreased compared to control. In contrast,when animals were subjected to the same intervention and subsequently were delivered near-term,prolonged LPS administration did not lead to structural lung abnormalities [26] ,suggesting a potential for repair and lung plasticity. A possible mechanism by which the effects of inflammation are altered is endotoxin tolerance [25] .
In a sheep model,repeated injections of LPS intraamniotically 7 days and 2 days before premature delivery inhibited lung cytokine production and minimized LPS-responsiveness of lung monocytes - depicted as decreased IL-6 production - compared to a single LPS exposure [27] . However,this effect is not limited to LPS as inducer of antenatal inflammation, but compromises other pro-inflammatory stimuli that bind to toll-like receptors (TLRs),which is referred to as cross-tolerance [28] . Lung and blood monocytes of fetal sheep exposed to repeated doses of intraamniotic LPS became tolerant to a variety of TLR-ligands like Pam3Cys (TLR1 and TLR2),flagellin (TLR5), CpG-DNA (TLR9) or LPS (TLR4) [29] . This study suggested that this effect was at least partially mediated by alterations in the common signalling pathway of different TLRs via interleukin-1 receptor associated kinases (IRAK) -4 and –M [29] . In sheep with chronic Ureaplasma colonization of the amniotic fluid,immune response on a subsequent acute stimulation with intraamniotic LPS was suppressed [30] .
In summary,the impact of the fetus’ innate immune response to chorioamnionitis on lung inflammation and injury is influenced by the complex interaction of different agents [24] . 3 Structural and inflammatory effects of maternal glucocorticoids
Prenatal administration of glucocorticosteroids (GCS) to the mother,evolved over the last decades to a standard therapy in cases of imminent premature birth [31] . The complex effects of GCS have been described as maturational effects on the fetal lung. Experimental approaches to the mechanisms of the beneficial treatment revealed interesting findings [32, 33] .
Lungs from sheep treated prenatally with GCS were functionally superior and histology and morphometry showed thinning of the primary septa. In addition, an increase in alveolar volume and a decrease in alveolar number were observed which suggested a simplification of alveolar structures [32] . Interestingly, in animal models this effect on lung gas volume, surfactant pool size and alveolar simplification equaled the effect of endotoxin [32] ,though endotoxin effects were not mediated by cortisol [33] .
Although prenatal steroids clinically proved to be safe in the presence of chorioamnionitis [34, 35] ,the combined effects of inflammation and potentially antiinflammatory glucocorticoids remain unclear. Animal experiments allow examination of interaction,time and dose dependence in contrast to clinical setting. In a sheep model,concomitant maternal betamethasone and intraamniotic endotoxin resulted in suppressed lung inflammation after one day,compared to LPS alone [36] . In contrast,5 days after this intervention lambs had signs of pulmonary inflammation and increased levels of proinflammatory cytokine mRNA [36] . The late increase in inflammation in the animals receiving both interventions suggested that glucocorticoids down regulated endotoxin-induced inflammation only on short notice and that this effect vanished after fetal clearance of the maternal glucocorticoids [36] . However,betamethasone 7 days before intraamniotic LPS exposure of fetal lambs suppressed the inflammatory response in the lung 7 days after LPS exposure,while betamethasone 7 days after LPS did not inhibit pulmonary inflammation at all [37] . In contrast,functional lung measurements revealed higher volumes when betamethasone was given after LPS,while administration at time of inflammation or before did not have such an effect [36, 37] . These findings highlight the complex interaction between inflammation and glucocorticoids in their influence on lung development and maturation in a time-dependent manner.
One key aspect of the direct effects of glucocorticoids and inflammation is their impact on maturation of surfactant production. Glucocorticoids alone increase surfactant protein-B (SP-B) mRNA [32] . In rabbit lung explants,IL-1α and dexamethasone additively increased the expression of SP-A and SP-B in early gestation. Later in gestation, SP-B and SP-C were suppressed by IL-1,whereas glucocorticoids tended to increase the expression of SP-B and SP-C and prevented the IL-1-induced suppression of SPs [38] . In the presence of LPS-induced chorioamnionitis,glucocorticoids had additional maturational effects on SP-D mRNA expression, saturated surfactant phosphatidylcholine and lung function,while steroids before LPS had no such effect [37] . 4 Impact on developmental signalling by GCS and inflammation
As mentioned before,both intraamniotic LPS exposure in fetal lambs [23] and prenatal treatment of pregnant sheep with GCS resulted in impaired alveolar development [32] . In order to differentiate the apparently similar effects of chorioamnionitis and prenatal steroid treatment,recent publications focused on targeting cellular mechanisms involved in both lung inflammation and maturation. A candidate being successfully linked to preterm lung alterations in both clinical and experimental setup is TGFβ1. TGFβ1 plays an essential for lung bud development [39] .
However,TGFβ is elevated in the lungs of preterm infants prior to BPD development [40] . While antenatal inflammation induced TGFβ1 expression in the lung of fetal lambs [41] ,prenatal GCS decreased TGFβ in BPD patients [42] . In a sheep model of chorioamnionitis,signaling downstream of TGFβ was induced by intrauterine inflammation,while antenatal GCS inhibited this activation [43] . Other signaling pathways contributing to lung development have also been proven to be influenced by intraamniotic inflammation,namely the Wingless-Int (Wnt) and Sonic Hedgehog (Shh) pathway [44] . Moreover,matrix metalloproteinases (MMP) have been shown to be required for normal lung development as they play a role in maintaining extracellular matrix homeostasis [45] .
Antenatal betamethasone treatment and LPS-induced chorioamnionitis in a fetal sheep model resulted in similar reduction of alveolar wall thickness,but with induction of different types of MMPs [45] . In summary, apparent structural similarities in lung maturation after inflammation and GCS might differ in “quality” and affected molecular pathways. Although there is a clear short-term benefit,both interventions might lead to simplification of alveoli,resulting in an aberrant lung structure. 5 Postnatal lung injury
Various postnatal insults contribute to lung inflammation and injury,including mechanical ventilation-induced trauma from volume and pressure changes,extension of the tissue and oxygen toxicity. Awareness of these therapy-associated damages grew amongst others from animal experiments,and resulted in adjustment of ventilation strategies. However,as indicated above,ventilation-associated lung injury might differ in the presence or absence of prenatal alterations priming the lung for a “second hit” [8] . 6 Perinatal resuscitation
Before starting mechanical ventilation,manual inflation of preterm babies (“bagging”) was a common procedure in the delivery room to “open” the liquid filled lung and to facilitate gas exchange. However,a study in preterm lambs revealed that the positive effect of surfactant replacement therapy was already compromised after six manual inflations directly after birth,depicted in decreased functional measurements and histologic signs of lung injury [46] .
In order to increase inspiratory volume and functional residual capacity (FRC) before starting ventilation in newborns,sustained inflation (SI) was extensively investigated in rabbits [47] and sheep [48] . While SI of 20 seconds increased FRC in preterm rabbits [47] and SI of 30 seconds improved recovery of circulation in near-term asphyxiated sheep [48] ,SI alone caused a modest increase in acute phase and pro-inflammatory cytokines in preterm sheep lungs [49] . However,in the same study SI did not alter the inflammation caused by continued ventilation [49] . 7 Ventilation trauma
In order to maintain lung volume,positive end expiratory pressure (PEEP) was introduced in ventilation strategies,having an impact on both lung function and lung inflammation: In surfactant-treated preterm lambs,ventilation with PEEP improved lung function [50] ,maintained surfactant pool size [50] and modulated the expression of proinflammatory mediators [51] .
When postnatally mechanical ventilation was established with high tidal volumes for only 15 min, this caused bronchial epithelial disruption in both large and small airways of preterm sheep [52] . In this model, it was demonstrated that cytokine production from stretch injury in the fetal sheep primarily derived from cells of the lung parenchyma instead of inflammatory cells [53] . In contrast,when surfactant depleted rabbits were ventilated with very low tidal volumes and consecutive hypercapnia,a decrease of pulmonary inflammation could be observed [53] . Utilizing high frequency oscillatory ventilation (HFOV) in an immature baboon model,which allows gas exchange with tidal volumes not exceeding death space,did not attenuate tracheal inflammation markers compared to conventional mechanical ventilation after 24 hours of ventilation,but in the subsequent recovery phase from RDS [54] . However,a recent meta-analysis of clinical trials failed to prove that HFOV was beneficial for preventing chronic lung disease in preterm infants [55] .
Current ventilation strategies aim to avoid mechanical ventilation in preterm infants by utilizing CPAP only with [56] or without [57] surfactant replacement. Results from animal experiments support this strategy: Prematurely delivered lambs treated with CPAP alone showed decreased levels of lung injury depicted by neutrophil counts in alveolar washes and reduced hydrogen peroxide in comparison to ventilated lambs [58] . In a baboon model of BPD, up to 28 days of nasal CPAP subsequent to 24 hours of mechanical ventilation did not result in arrested alveolar development in contrast to previously ventilated baboons [59] .
These findings indicate that mechanical ventilation influences both inflammatory processes and mechanisms involved in postnatal developmental signalling in the lung which is supported by a recent experiment,where adverse effects on alveolarization were described in mechanically ventilated preterm, but not in term lambs [60] . 8 The second hit — how do pre- and postnatal insults add?
If pro-inflammatory effects of ventilation add to prenatally acquired inflammation is controversial. Conflicting data exists in a rat lung explant model. While Tremblay et al [61] showed that mechanical ventilation alone significantly influenced the inflammatory environment of the lung,Ricard et al [62] only found significant amounts of pro-inflammatory cytokines in the bronchoalveolar lavage fluid (BALF) of rats previously injected with LPS,however irrespective of normal vs. injurious ventilation. In a term sheep model, intratracheal endotoxin administration caused lung inflammation after 6 hours of ventilation,but had no systemic effects. In contrast,intratracheal LPS combined with high tidal volume ventilation caused both lung and systemic inflammation,even with 100 times lower doses of LPS [63] . However,cytokine markers in the lung subsequent to intratracheal LPS administration were increased compared to saline,irrespective if lambs were mechanically ventilated or received respiratory support (continuous positive airway pressure, CPAP) [64] . Additionally,antenatal colonization of sheep fetuses with Ureaplasma did not change lung function or modulate the lung injury and inflammation caused by high tidal volume ventilation [65] . However,pro-inflammatory effects mediated by ventilation may be counteracted by an “anti-inflammatory first hit”: Antenatal betamethasone proved to be beneficial when preterm lambs were resuscitated with escalating tidal volumes directly after birth [66] . In this model,antenatal betamethasone decreased lung injury without decreasing lung inflammatory cells or systemic acute phase responses [66] . 9 Inflammation and sepsis
While systemic inflammation might result from pulmonary inflammation,it may also precede pulmonary alterations. Lambs given 5 µg/kg endotoxin intravenously showed decreased gas exchange, and both lung function and systemic inflammatory response were comparable to animals which received high doses of LPS intratracheally in combination with high tidal volume ventilation [63] . Furthermore,shortterm exposure to systemic fetal inflammation led to lung maturation and disturbed lung structure [67] , indicating the strong link between lung injury and systemic inflammation including early onset sepsis. Recently,the effects of postnatal intravenous LPS were assessed in the context of antenatal exposure to chorioamnionitis [68] . Intravenous LPS exposure increased levels of inflammatory cytokine mRNA in the lung of ventilated lambs. However,in combination with antenatal exposure to chorioamnionitis,this effect varied between cytokines. In addition,endotoxemia resulted in higher oxygen requirement if animals were antenatally exposed to chorioamnionitis,while the adverse effects of endotoxemia on the blood pressure were prevented [68] . 10 Oxygen — a multifaceted drug
Being the longest established therapeutic option for respiratory distress of preterm infants, recent clinical studies confirmed an ambiguous role of oxygen supplementation [69, 70] . Clinical studies showed a close relationship between oxygen and the development or exacerbation of BPD [71, 72] . Growing evidence suggests that reactive oxygen species interact with cell growth and development [73] . This cumulated in the hypothesis of an oxygen radical disease (ORD) of neonatology as one disease with various conditions such as BPD,necrotizing enterocolitis (NEC), retinopathy of prematurity (ROP) and periventricular leucomalacia (PVL) [73] . Although a common entity of these conditions has not been proven so far,clinical and experimental studies indicate that oxidative stress in the first days of life is involved in the development of BPD [73] ,partially because preterm infants have lower anti-oxidative capacity [74] .
However,the interaction between oxygen and lung injury is complex. In a mouse model,neonatal hyperoxia resulted in histological signs of lung injury and decreased lung function [75] . Concomitantly,levels of mRNA of proinflammatory cytokines like IL-1α were increased in the lung [75] . In a rabbit model, hyperoxia enhanced inflammatory cytokine response of alveolar macrophages of preterm animals in contrast to term animals [76] . In this model,hyperoxia significantly increased intracellular oxygen radical content only in premature macrophages [76] .
Beside exposure to high concentrations of inhaled oxygen,preterm infants might be challenged by oxidative stress resulting from inflammatory processes which might occur already in utero [73] . A recent clinical study related oxygen radical exposure of the fetus to neonatal outcome of preterm babies by measuring lipid peroxidation by isoprostane levels in umbilical cord venous blood. Isoprostane levels correlated with mortality and the risk of developing one or more ORD,but not with BPD alone [77] . Experimentally,protein carbonyls as marker of oxidative stress increased in the airways of sheep fetuses exposed to 7 days LPS,but not after 2 days of exposure [78] . Regarding anti-oxidative capacity,lung antioxidant enzyme activity was not altered when IL-1α was administered intraamniotically to preterm sheep [12] ,whereas the antioxidant enzyme activity in the fetal lung of preterm sheep exposed to LPS 7 days before delivery increased in a dose dependent manner [79] . In addition,activity of different antioxidant enzymes increased at different points in time after LPS exposure [79] .
In summary,interaction between prenatal and postnatal contributors to lung injury exceeds “simple” collaboration,but occurs in a complex pattern. 11 Postnatal modulators of lung injury
Experimental studies also allow assessment of postnatal therapeutic strategies on acute and chronic lung injury. The development of surfactant replacement therapy was a hallmark in RDS therapy, but might be less effective in infants with severe chorioamnionitis,associated with increased BPD susceptibility [80] . Surfactant inactivation by proteins leaking into the alveoli due to inflammation or stretch injury might be overcome by synthetic surfactants designed to resist inactivation,as shown in a sheep model [81] . In addition,experimental data suggest an anti-inflammatory potential of surfactant components [82] . Eventually,positive effects of prenatal GCS on endogenous surfactant production and maturation are supported postnatally by drugs like caffeine both in vitroand in a sheep model [83, 84] .
Clinical studies suggesting positive effects of other drugs on lung injury need to be substantiated by experimental data. Vitamin A,which recently showed positive effects on preventing BPD in preterm infants in a clinical study [85] ,experimentally resulted in decreased lung fibrosis in rats [86] and increased lung elastin expression in ventilated premature baboons [87] .
Recently,it was demonstrated that intraamniotic LPS disrupted the Shh pathway in the lungs of preterm lambs [44] . Vitamin A is decreased in fetal sheep lungs after intraamniotic LPS [88] . The beneficial effect of vitamin A on BPD development may therefore be explained in part by its induction of the Shh signaling pathway.
This is a good example how experimental data supports the knowledge about therapeutic interventions. In contrast,experimental data examining adverse effects lay the essential basis for clinical trials,especially when clinical data cannot resolve uncertainty regarding therapeutic merits [89] .
Postnatal steroid therapy with dexamethasone,which has proven effectiveness in clinical trials both early and late after birth [90, 91] ,was associated with adverse neurocognitive outcomes when administered early [92] .
Alternatively,hydrocortisone may be better tolerated in neonates [93] as it showed little effect on somatic growth and pulmonary outcome [94] and no negative effects on hippocampal function in a rat model [95] .
Clinical trials may help to determine the efficacy and safety of postnatal hydrocortisone administration to ventilator dependent preterm infants [96] . The beneficial role of experimental models was recently highlighted in the development of stem cell based therapies for BPD [97] . 12 Outcome and long term effects
Although growing experimental evidence elucidate the link between lung injury,lung inflammation,repair and altered lung development, the interactions between injurious insults and inflammatory stimuli on different levels are complex and remain to be completely understood. Continuous advances in the therapy of the immature infant partially resolve the problems derived from acute and chronic lung injury,but create new problems regarding pulmonary outcome of these infants. Furthermore,recent findings support the hypothesis that chronic lung injury originating in this early period of life or even antenatally may indeed have long-term adverse respiratory effects [98] . Kumar and colleagues [99] recently reported an association between chorioamnionitis and both recurrent wheezing and physician-diagnosed asthma. In addition,young adult survivors of moderate and severe bronchopulmonary dysplasia may be left with residual functional and characteristic structural pulmonary abnormalities, most notably emphysema [100] .
Therefore,long term follow-up of infants born prematurely regarding frequency and quality of their pulmonary impairment as provided by birth cohort studies are urgently needed [101] . Once the relative impact of distinct early life factors on pulmonary outcome are identified,carefully planned animal experiments will provide further insight into related mechanisms on all levels,including inflammatory alterations,developmental changes and the impact of early interventions,and will allow testing innovative preventive and therapeutic approaches. Thereby they will form a stable foundation to plan clinical trials which will help to improve pulmonary outcome in the neonatal period and beyond. 附中文概要(急性和慢性肺损伤的模式及病因:基于非临床性实验证据的剖析)
满足最基本需求的肺功能是早产儿存活的先决条件。早产儿肺结构和功能欠成熟,常常伴发呼吸窘迫综合征(RDS)。 除结构不成熟之外,早产儿肺组织还易因出生前后各种不利状况及因素而受损,进而防碍生后肺部的正常发育,促发非成 熟性慢性肺部病变,导致支气管肺发育不良(BPD)。该文将以由炎症变化而引发并与炎症变化密切相关的肺损伤为重点, 全面综述用以探讨早产儿肺部炎症反应机制的各种实验模型,这其中包括用来研究绒毛膜羊膜炎发病机制的模型。人们知道, 绒毛膜羊膜炎与早产密切关。从临床角度讲,胎儿宫内接触绒毛膜羊膜炎会导致胎儿肺组织发育异常,进而影响早产儿的 肺功能。根据临床数据,有作者认为:暴露于绒毛膜羊膜炎的胎儿其支气管肺发育不良的风险增高,但 RDS 的风险反而降 低。目前有实验数据表明,急性和慢性羊膜炎均可导致胎儿肺部炎症反应。一些研究人员建立了各种动物模型,他们除了 借助这些模型来检测肺组织中诸如 IL-1 之类炎症介质的水平外,他们还用这些模型研究过炎症介质在肺发育成熟过程中的 作用。在接触过宫内炎症的动物胎儿,肺组织磷脂酰胆碱的生成量及表面活性蛋白的水平增高,这表明脂酰胆碱表面活性 蛋白会影响肺上皮细胞成熟,其影响程度之大小主要取决于暴露炎症因子的时间和剂量以及炎症因子的性质。使用细菌脂 多糖(LPS)或解脲活性物作为炎症因子处理实验动物可以模拟人绒毛膜羊膜炎的临床表现。此外,实验数据表明,胎儿 似乎具有抗衡并降低炎症反应程度的能力。目前,越来越多的证据表明抗逆内毒素是胎儿炎症调节的一个重要机制。 产前 用糖皮质激素(GCS)处理孕妇是早产的标准治疗方案,该处理可对胎儿肺组织和免疫细胞的成熟施以复杂的影响。有趣 的是,GCS 对肺形态和功能的作用与 LPS 诱导的宫内羊膜炎症反应雷同。然而,动物实验显示,虽然在动物炎症反应和孕 妇 GCS 处理后肺器官在成熟过程度中其构成变化极其相似,但两者存在“质”上的不同,且涉及的分子途径也各异。比如, 产前炎症可诱发胎儿肺组织的 TGFβ 表达增高并激活其下游信号通路,然而产前 GCS 处理孕妇的效果则相反。尽管这两种 干预都有短期益效,但他们均可使肺泡简化,进而导致肺结构异常。在肺发育成熟过程中,即便倍他米松和 LPS 均能类似 程度地减少肺泡壁的厚度,但孕妇产前倍他米松治疗和动物产前 LPS 诱导动物绒毛膜羊膜炎所激活的其他信号传导通路 (比 如基质金属蛋白酶 MMP)也各不相同。此外,产后治疗干预可能会导致肺部炎症和损伤。包括动物实验在内的诸多研究结 果使人们越来越清楚地意识到与药物治疗相关的损伤,进而也对临床治疗方案作过适当的调整。迄今,不少研究人员通过 各种动物模型对围产期复苏时子宫炎症对胎儿肺功能和肺感染的作用进行过广泛的研究。比如在机械通气方面,有关呼气 末正压 (PEEP)对肺功能、肺表面活性剂储存以及肺部炎症介质的正面作用就有不少来自动物模型的研究报道。这些动物 模型实验探讨过诸如高频振动通气(HFOV)之类的机械通气技术对肺部的损害作用,其结果支持无论有无表面活性剂替 代治疗都应避免机械通气的新治疗理念。补氧可进一步损伤肺组织并引发炎症。越来越多的证据表明,活性氧与细胞生长 和发育有交互影响。据此,近年来便产生了下面的假说:支气管肺发育不良是一种由氧自由基所引发的病变。动物实验数 据业己揭示氧和肺损伤之间的复杂交互作用,然而其机制尚不十分清楚。产前炎症可能早已引发胎儿肺部氧化应激反应并 改变抗氧化能力,这就使得氧气与肺损伤之间的复杂关系变得更为复杂。此外,有动物实验就广泛用于新生婴儿的药物如 类固醇、表面活性剂、咖啡因和维生素 A 对肺部的炎症变化及损伤作用进行过试验。这些实验数据可作为临床试验的依据, 尤其在临床数据无法解决有关治疗效果或机制的不确定性情况下更是如此。这方面的一个典型例子就是那个有关慢性肺损 伤进展差异原因令人印象深刻、支持“二次打击”假说的附加解释。临床研究暗示早产婴儿生前炎症和生后机械通气可能 对支气管发育不良有协同作用。然而,诸如炎症之类的围产期病变和产后不同治疗干预之间的协同效应仍有待进一步确定。 精心设计的实验研究将有助于阐明肺损伤、肺部炎症、肺修复及非正常肺发育之间的复杂关系,同时也将有助于确立源于 新生婴儿早期肺部发育变异与终身性呼吸功能障碍之间的联系。
| [1] | Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery[J]. N Engl J Med, 2000, 342(20): 1500-1507. |
| [2] | Gantert M, Been JV, Gavilanes AW, et al. Chorioamnionitis: a multiorgan disease of the fetus? [J]. J Perinatol, 2010, 30 (Suppl): S21-S30. |
| [3] | Lahra MM, Beeby PJ, Jeffery HE. Maternal versus fetal inflammation and respiratory distress syndrome: a 10-year hospital cohort study[J]. Arch Dis Child Fetal Neonatal Ed, 2009, 94(1): F13-16. |
| [4] | Been JV, Zimmermann LJ. Histological chorioamnionitis and respiratory outcome in preterm infants[J]. Arch Dis Child Fetal Neonatal Ed, 2009, 94(3): F218-225. |
| [5] | Watterberg KL, Demers LM, Scott SM, et al. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops[J]. Pediatrics, 1996, 97(2): 210-215. |
| [6] | Jobe AH. What is RDS in 2012? [J]. Early Hum Dev, 2012, 88 (Suppl 2): S42-S44. |
| [7] | Jobe AH. What is BPD in 2012 and what will BPD become? [J]. Early Hum Dev, 2012, 88 (Suppl 2): S27-S28. |
| [8] | Van Marter LJ, Dammann O, Allred EN, et al. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants[J]. J Pediatr, 2002, 140(2): 171-176. |
| [9] | Kunzmann S, Collins JJP, Kuypers E, et al. Thrown off balance: the effect of antenatal inflammation on the developing lung and immune system[J]. Am J Obstet Gynecol, 2013, 208(6): 429-437. |
| [10] | Bry K, Lappalainen U, Hallman M. Intraamniotic interleukin-1 accelerates surfactant protein synthesis in fetal rabbits and improves lung stability after premature birth[J]. J Clin Invest, 1997, 99(12): 2992-2999. |
| [11] | Willet KE, Kramer BW, Kallapur SG, et al. Intra-amniotic injection of IL-1 induces inflammation and maturation in fetal sheep lung[J]. Am J Physiol Lung Cell Mol Physiol, 2002, 282(3): L411-420. |
| [12] | Sosenko IR, Kallapur SG, Nitsos I, et al. IL-1 alpha causes lung inflammation and maturation by direct effects on preterm fetal lamb lungs[J]. Pediatr Res, 2006 60(3): 294-298. |
| [13] | Glumoff V, Vayrynen O, Kangas T, et al. Degree of lung maturity determines the direction of the interleukin-1- induced effect on the expression of surfactant proteins[J]. Am J Respir Cell Mol Biol, 2000, 22(3): 280-228. |
| [14] | Kallapur SG, Nitsos I, Moss TJ, et al. IL-1 mediates pulmonary and systemic inflammatory responses to chorioamnionitis induced by lipopolysaccharide[J]. Am J Respir Crit Care Med, 2009, 179(10): 955-961. |
| [15] | Kramer BW, Moss TJ, Willet KE, et al. Dose and time response after intraamniotic endotoxin in preterm lambs[J]. Am J Respir Crit Care Med, 2001, 164(6): 982-988. |
| [16] | Kallapur SG, Willet KE, Jobe AH, et al. Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs[J]. Am J Physiol Lung Cell Mol Physiol, 2001, 280(3): L527-L536. |
| [17] | Kallapur SG, Kramer BW, Jobe AH. Ureaplasma and BPD[J]. Semin Perinatol, 2013, 37(2): 94-101. |
| [18] | DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation[J]. PLoS One, 2008, 3(8): e3056. |
| [19] | Kotecha S, Hodge R, Schaber JA, et al. Pulmonary Ureaplasma urealyticum is associated with the development of acute lung inflammation and chronic lung disease in preterm infants[J]. Pediatr Res, 2004, 55(1): 61-68. |
| [20] | Collins JJ, Kallapur SG, Knox CL, et al. Inflammation in fetal sheep from intra-amniotic injection of Ureaplasma parvum[J]. Am J Physiol Lung Cell Mol Physiol, 2010, 299(6): L852-L860. |
| [21] | Polglase GR, Dalton RG, Nitsos I, et al. Pulmonary vascular and alveolar development in preterm lambs chronically colonized with Ureaplasma parvum[J]. Am J Physiol Lung Cell Mol Physiol, 2010, 299(2): L232-L241. |
| [22] | Moss TJ, Knox CL, Kallapur SG, et al. Experimental amniotic fluid infection in sheep: effects of Ureaplasma parvum serovars 3 and 6 on preterm or term fetal sheep[J]. Am J Obstet Gynecol, 2008, 198(1): 122 e1-8. |
| [23] | Moss TJ, Newnham JP, Willett KE, et al. Early gestational intra-amniotic endotoxin: lung function, surfactant, and morphometry[J]. Am J Respir Crit Care Med, 2002, 165(6): 805-811. |
| [24] | Kramer BW, Kallapur S, Newnham J, et al. Prenatal inflammation and lung development[J]. Semin Fetal Neonatal Med, 2009, 14(1): 2-7. |
| [25] | Kramer BW, Jobe AH. The clever fetus: responding to inflammation to minimize lung injury[J]. Biol Neonate, 2005, 88(3): 202-207. |
| [26] | Kallapur SG, Nitsos I, Moss TJ, et al. Chronic endotoxin exposure does not cause sustained structural abnormalities in the fetal sheep lungs[J]. Am J Physiol Lung Cell Mol Physiol, 2005, 288(5): L966-L974. |
| [27] | Kallapur SG, Jobe AH, Ball MK, et al. Pulmonary and systemic endotoxin tolerance in preterm fetal sheep exposed to chorioamnionitis[J]. J Immunol, 2007, 179(12): 8491-8499. |
| [28] | Kramer BW. Chorioamnionitis-new ideas from experimental models[J]. Neonatology, 2011, 99(4): 320-325. |
| [29] | Kramer BW, Kallapur SG, Moss TJ, et al. Intra-amniotic LPS modulation of TLR signaling in lung and blood monocytes of fetal sheep[J]. Innate Immun, 2009, 15(2): 101-107. |
| [30] | Kallapur SG, Kramer BW, Knox CL, et al. Chronic fetal exposure to Ureaplasma parvum suppresses innate immune responses in sheep[J]. J Immunol, 2011, 187(5): 2688-2695. |
| [31] | Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes[J]. Jama, 1995, 273(5): 413-418. |
| [32] | Willet KE, Jobe AH, Ikegami M. Antenatal endotoxin and glucocorticoid effects on lung morphometry in preterm lambs[J]. Pediatr Res, 2000, 48(6): 782-788. |
| [33] | Jobe AH, Newnham JP, Willet KE, et al. Endotoxin-induced lung maturation in preterm lambs is not mediated by cortisol[J]. Am J Respir Crit Care Med, 2000, 162(5): 1656-1661. |
| [34] | Goldenberg RL, Andrews WW, Faye-Petersen OM, et al. The Alabama preterm birth study: corticosteroids and neonatal outcomes in 23- to 32-week newborns with various markers of intrauterine infection[J]. Am J Obstet Gynecol, 2006, 195(4): 1020-1024. |
| [35] | Been JV, Degraeuwe PL, Kramer BW, et al. Antenatal steroids and neonatal outcome after chorioamnionitis: a meta-analysis[J]. Bjog, 2011, 118(2): 113-122. |
| [36] | Kallapur SG, Kramer BW, Moss TJ, et al. Maternal glucocorticoids increase endotoxin-induced lung inflammation in preterm lambs[J]. Am J Physiol Lung Cell Mol Physiol, 2003, 284(4): L633-L642. |
| [37] | Kuypers E, Collins JJ, Kramer BW, et al. Intra-amniotic LPS and antenatal betamethasone: inflammation and maturation in preterm lamb lungs[J]. Am J Physiol Lung Cell Mol Physiol, 2012, 302(4): L380-L389. |
| [38] | Vayrynen O, Glumoff V, Hallman M. Inflammatory and anti-inflammatory responsiveness of surfactant proteins in fetal and neonatal rabbit lung[J]. Pediatr Res, 2004, 55(1): 55-60. |
| [39] | Bartram U, Speer CP. The role of transforming growth factor beta in lung development and disease[J]. Chest, 2004, 125(2): 754-765. |
| [40] | Kotecha S, Wangoo A, Silverman M, et al. Increase in the concentration of transforming growth factor beta-1 in bronchoalveolar lavage fluid before development of chronic lung disease of prematurity[J]. J Pediatr, 1996, 128(4): 464-469. |
| [41] | Kunzmann S, Speer CP, Jobe AH, et al. Antenatal inflammation induced TGF-beta1 but suppressed CTGF in preterm lungs[J]. Am J Physiol Lung Cell Mol Physiol, 2007, 292(1): L223-L231. |
| [42] | Lecart C, Cayabyab R, Buckley S, et al. Bioactive transforming growth factor-beta in the lungs of extremely low birthweight neonates predicts the need for home oxygen supplementation[J]. Biol Neonate, 2000, 77(4): 217-223. |
| [43] | Collins JJ, Kunzmann S, Kuypers E, et al. Antenatal glucocorticoids counteract LPS changes in TGF-beta pathway and caveolin-1 in ovine fetal lung[J]. Am J Physiol Lung Cell Mol Physiol, 2013, 304(6): L438-L444. |
| [44] | Collins JJ, Kuypers E, Nitsos I, et al. LPS-induced chorioamnionitis and antenatal corticosteroids modulate Shh signaling in the ovine fetal lung[J]. Am J Physiol Lung Cell Mol Physiol, 2012, 303(9): L778-L787. |
| [45] | Sweet DG, Huggett MT, Warner JA, et al. Maternal betamethasone and chorioamnionitis induce different collagenases during lung maturation in fetal sheep[J]. Neonatology, 2008, 94(2): 79-86. |
| [46] | Bjorklund LJ, Ingimarsson J, Curstedt T, et al. Manual ventilation with a few large breaths at birth compromises the therapeutic effect of subsequent surfactant replacement in immature lambs[J]. Pediatr Res, 1997, 42(3):348-355. |
| [47] | te Pas AB, Siew M, Wallace MJ, et al. Effect of sustained inflation length on establishing functional residual capacity at birth in ventilated premature rabbits[J]. Pediatr Res, 2009, 66(3): 295-300. |
| [48] | Klingenberg C, Sobotka KS, Ong T, et al. Effect of sustained inflation duration; resuscitation of near-term asphyxiated lambs[J]. Arch Dis Child Fetal Neonatal Ed, 2013, 98(3): F222-F227. |
| [49] | Hillman NH, Kemp MW, Noble PB, et al. Sustained Inflation at birth did not protect preterm fetal sheep from lung injury[J]. Am J Physiol Lung Cell Mol Physiol, 2013, 305(6): L446-L453. |
| [50] | Michna J, Jobe AH, Ikegami M. Positive end-expiratory pressure preserves surfactant function in preterm lambs[J]. Am J Respir Crit Care Med, 1999, 160(2): 634-649. |
| [51] | Naik AS, Kallapur SG, Bachurski CJ, et al. Effects of ventilation with different positive end-expiratory pressures on cytokine expression in the preterm lamb lung[J]. Am J Respir Crit Care Med, 2001, 164(3): 494-498. |
| [52] | Hillman NH, Kallapur SG, Pillow JJ, et al. Airway injury from initiating ventilation in preterm sheep[J]. Pediatr Res, 2010, 67(1): 60-65. |
| [53] | Fuchs H, Mendler MR, Scharnbeck D, et al. Very low tidal volume ventilation with associated hypercapnia--effects on lung injury in a model for acute respiratory distress syndrome[J]. PLoS One, 2011, 6(8): e23816. |
| [54] | Yoder BA, Siler-Khodr T, Winter VT, et al. High-frequency oscillatory ventilation: effects on lung function, mechanics, and airway cytokines in the immature baboon model for neonatal chronic lung disease[J]. Am J Respir Crit Care Med, 2000, 162(5): 1867-1876. |
| [55] | Cools F, Askie LM, Offringa M, et al. Elective high-frequency oscillatory versus conventional ventilation in preterm infants: a systematic review and meta-analysis of individual patients' data[J]. Lancet, 2010, 375(9731): 2082-2091. |
| [56] | Gopel W, Kribs A, Ziegler A, et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial[J]. Lancet, 2011, 378(9803): 1627-1634. |
| [57] | Gittermann MK, Fusch C, Gittermann AR, et al. Early nasal continuous positive airway pressure treatment reduces the need for intubation in very low birth weight infants[J]. Eur J Pediatr, 1997, 156(5): 384-388. |
| [58] | Jobe AH, Kramer BW, Moss TJ, et al. Decreased indicators of lung injury with continuous positive expiratory pressure in preterm lambs[J]. Pediatr Res, 2002, 52(3): 387-392. |
| [59] | Thomson MA, Yoder BA, Winter VT, et al. Treatment of immature baboons for 28 days with early nasal continuous positive airway pressure[J]. Am J Respir Crit Care Med, 2004, 169(9): 1054-1062. |
| [60] | Bland RD, Xu L, Ertsey R, et al. Dysregulation of pulmonary elastin synthesis and assembly in preterm lambs with chronic lung disease[J]. Am J Physiol Lung Cell Mol Physiol, 2007, 292(6): L1370-L1384. |
| [61] | Tremblay L, Valenza F, Ribeiro SP, et al. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model[J]. J Clin Invest, 1997, 99(5): 944-952. |
| [62] | Ricard JD, Dreyfuss D, Saumon G. Production of inflammatory cytokines in ventilator-induced lung injury: a reappraisal[J]. Am J Respir Crit Care Med, 2001, 163(5): 1176-1180. |
| [63] | Kramer BW, Kramer S, Ikegami M, et al. Injury, inflammation, and remodeling in fetal sheep lung after intra-amniotic endotoxin[J]. Am J Physiol Lung Cell Mol Physiol, 2002, 283(2): L452-L459. |
| [64] | Polglase GR, Hillman NH, Ball MK, et al. Lung and systemic inflammation in preterm lambs on continuous positive airway pressure or conventional ventilation[J]. Pediatr Res, 2009, 65(1): 67-71. |
| [65] | Polglase GR, Hillman NH, Pillow JJ, et al. Ventilation-mediated injury after preterm delivery of Ureaplasma parvum colonized fetal lambs[J]. Pediatr Res, 2010, 67(6): 630-635. |
| [66] | Hillman NH, Pillow JJ, Ball MK, et al. Antenatal and postnatal corticosteroid and resuscitation induced lung injury in preterm sheep[J]. Respir Res, 2009, 10: 124. |
| [67] | Kramer BW, Ladenburger A, Kunzmann S, et al. Intravenous lipopolysaccharide-induced pulmonary maturation and structural changes in fetal sheep[J]. Am J Obstet Gynecol, 2009, 200(2): 195 e1-10. |
| [68] | Gisslen T, Hillman NH, Musk GC, et al. Repeated exposure to intra-amniotic LPS partially protects against adverse effects of intravenous LPS in preterm lambs[J]. Innate Immun, 2013, 20(2): 214-224. |
| [69] | Carlo WA, Finer NN, Walsh MC, et al. Target ranges of oxygen saturation in extremely preterm infants[J]. N Engl J Med, 2010, 362(21): 1959-1969. |
| [70] | Stenson B, Brocklehurst P, Tarnow-Mordi W. Increased 36-week survival with high oxygen saturation target in extremely preterm infants[J]. N Engl J Med, 2011, 364(17): 1680-1682. |
| [71] | Supplemental Therapeutic Oxygen for Prethreshold Retinopathy Of Prematurity (STOP-ROP), a randomized, controlled trial. I: primary outcomes[J]. Pediatrics, 2000, 105(2): 295-310. |
| [72] | Van Marter LJ, Allred EN, Pagano M, et al. Do clinical markers of barotrauma and oxygen toxicity explain interhospital variation in rates of chronic lung disease? The Neonatology Committee for the Developmental Network[J]. Pediatrics, 2000, 105(6): 1194-1201. |
| [73] | Saugstad OD. Update on oxygen radical disease in neonatology[J]. Curr Opin Obstet Gynecol, 2001, 13(2): 147-153. |
| [74] | Rogers S, Witz G, Anwar M, et al. Antioxidant capacity and oxygen radical diseases in the preterm newborn[J]. Arch Pediatr Adolesc Med, 2000, 154(6): 544-548. |
| [75] | Warner BB, Stuart LA, Papes RA, et al. Functional and pathological effects of prolonged hyperoxia in neonatal mice[J]. Am J Physiol, 1998, 275(1 Pt 1): L110-L117. |
| [76] | Rozycki HJ, Comber PG, Huff TF. Cytokines and oxygen radicals after hyperoxia in preterm and term alveolar macrophages[J]. Am J Physiol Lung Cell Mol Physiol, 2002, 282(6): L1222-L1228. |
| [77] | Weinberger B, Nisar S, Anwar M, Ostfeld B, Hegyi T. Lipid peroxidation in cord blood and neonatal outcome[J]. Pediatr Int, 2006, 48(5): 479-483. |
| [78] | Cheah FC, Jobe AH, Moss TJ, et al. Oxidative stress in fetal lambs exposed to intra-amniotic endotoxin in a chorioamnionitis model[J]. Pediatr Res, 2008, 63(3): 274-279. |
| [79] | Sosenko IR, Jobe AH. Intraamniotic endotoxin increases lung antioxidant enzyme activity in preterm lambs[J]. Pediatr Res, 2003, 53(4): 679-683. |
| [80] | Been JV, Rours IG, Kornelisse RF, et al. Chorioamnionitis alters the response to surfactant in preterm infants[J]. J Pediatr, 2010, 156(1): 10-5 e1. |
| [81] | Seehase M, Collins JJ, Kuypers E, et al. New surfactant with SP-B and C analogs gives survival benefit after inactivation in preterm lambs[J]. PLoS One, 2012, 7(10): e47631. |
| [82] | Gille C, Spring B, Bernhard W, et al. Differential effect of surfactant and its saturated phosphatidylcholines on human blood macrophages[J]. J Lipid Res, 2007, 48(2): 307-317. |
| [83] | Fehrholz M, Bersani I, Kramer BW, et al. Synergistic effect of caffeine and glucocorticoids on expression of surfactant protein B (SP-B) mRNA[J]. PLoS One, 2012, 7(12): e51575. |
| [84] | Fehrholz M, Hutten M, Kramer BW, et al. Amplification of steroid-mediated SP-B expression by physiological levels of caffeine[J]. Am J Physiol Lung Cell Mol Physiol, 2014,306(1): L101-L109. |
| [85] | Moreira A, Caskey M, Fonseca R, et al. Impact of providing vitamin A to the routine pulmonary care of extremely low birth weight infants[J]. J Matern Fetal Neona, 2012, 25(1): 84-88. |
| [86] | Ozer EA, Kumral A, Ozer E, et al. Effect of retinoic acid on oxygen-induced lung injury in the newborn rat[J]. Pediatr Pulmonol, 2005, 39(1): 35-40. |
| [87] | Pierce RA, Joyce B, Officer S, et al. Retinoids increase lung elastin expression but fail to alter morphology or angiogenesis genes in premature ventilated baboons[J]. Pediatr Res, 2007, 61(6): 703-709. |
| [88] | Kramer BW, Albertine KH, Moss TJ, et al. All-trans retinoic acid and intra-amniotic endotoxin-mediated effects on fetal sheep lung[J]. Anat Rec (Hoboken), 2008, 291(10): 1271-1277. |
| [89] | Eichenwald EC, Stark AR. Are postnatal steroids ever justified to treat severe bronchopulmonary dysplasia?[J]. Arch Dis Child Fetal Neonatal Ed, 2007, 92(5): F334- F337. |
| [90] | Halliday HL, Ehrenkranz RA, Doyle LW. Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants[J]. Cochrane Database Syst Rev, 2009, (1):CD001146. |
| [91] | Halliday HL, Ehrenkranz RA, Doyle LW. Late (>7 days) postnatal corticosteroids for chronic lung disease in preterm infants[J]. Cochrane Database Syst Rev, 2009(1):CD001145. |
| [92] | Barrington KJ. The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs[J]. BMC Pediatr, 2001, 1:1. |
| [93] | Watterberg KL, Shaffer ML, Mishefske MJ, et al. Growth and neurodevelopmental outcomes after early low-dose hydrocortisone treatment in extremely low birth weight infants[J]. Pediatrics, 2007, 120(1): 40-48. |
| [94] | Lee HJ, Kim BI, Choi ES, et al. Effects of postnatal dexamethasone or hydrocortisone in a rat model of antenatal lipopolysaccharide and neonatal hyperoxia exposure[J]. J Korean Med Sci, 2012, 27(4): 395-401. |
| [95] | Huang CC, Lin HR, Liang YC, et al. Effects of neonatal corticosteroid treatment on hippocampal synaptic function[J]. Pediatr Res, 2007, 62(3): 267-270. |
| [96] | Onland W, Offringa M, Cools F, et al. Systemic Hydrocortisone To Prevent Bronchopulmonary Dysplasia in preterm infants (the SToP-BPD study); a multicenter randomized placebo controlled trial[J]. BMC Pediatr, 2011, 11:102. |
| [97] | Fung ME, Thebaud B. Stem cell-based therapy for neonatal lung disease-it's in the juice[J]. Pediatr Res, 2014, 75(1-1): 2-7. |
| [98] | Brostrom EB, Akre O, Katz-Salamon M, et al. Obstructive pulmonary disease in old age among individuals born preterm[J]. Eur J Epidemiol, 2013, 28(1): 79-85. |
| [99] | Kumar R, Yu Y, Story RE, et al. Prematurity, chorioamnionitis, and the development of recurrent wheezing: a prospective birth cohort study[J]. J Allergy Clin Immunol, 2008, 121(4): 878-884. |
| [100] | Wong PM, Lees AN, Louw J, et al. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia[J]. Eur Respir J, 2008, 32(2): 321-328. |
| [101] | Larsen PS, Kamper-Jorgensen M, Adamson A, et al. Pregnancy and birth cohort resources in europe: a large opportunity for aetiological child health research[J]. Paediatr Perinat Epidemiol, 2013, 27(4): 393-414. |
 2014, Vol. 16
2014, Vol. 16


