认知受损和焦虑抑郁情绪障碍是癫癎患者面临的普遍而重要的并发症。国内外研究报道,1/3的癫癎患者有认知缺陷以及焦虑抑郁情绪障碍[1-2]。认知受损是癫癎致残的最严重后果之一,而焦虑抑郁情绪可以加重认知功能障碍,是癫癎患者生活质量恶化的较强预警因素[3]。目前国内对于成人癫癎的认知及焦虑抑郁情绪障碍有较多研究,但对于儿童癫癎此类研究较少。
颞叶癫癎及特发性癫癎均是儿童及青少年中较常见的癫癎综合征[4]。而颞叶癫癎患者是认知受损及合并焦虑抑郁情绪障碍的高危人群[5-7]。据报道特发性癫癎患儿也存在注意力缺陷以及记忆、学习等认知功能受损[8]。研究发现,诸多因素影响癫癎患者认知功能,主要包括病因、起病年龄、病程、发作类型、发作频率、发作间期癫癎样放电、抗癫癎药物及文化程度等[9-14]。因此,临床上对癫癎患者进行认知功能评估并深入分析影响认知功能的危险因素对于如何早期干预、提高患儿生活质量、改善预后具有重要意义。本研究将采用一系列神经心理测评量表评估颞叶癫癎及特发性癫癎患儿的认知功能及焦虑抑郁情绪,并分析影响认知功能的危险因素。
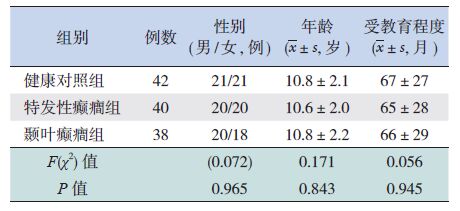
1 资料与方法 1.1 病例选择随机选取2014年9 月至 2016 年 4 月中南大学湘雅医院癫癎专科门诊确诊为颞叶癫癎(38例)及特发性癫癎(40例)的8 ~14 岁儿童作为研究对象。颞叶癫癎组平均10.8±2.2岁,特发性癫癎组平均10.6±2.0岁。并随机选取42 例年龄、性别、文化程度与病例组相匹配的体检儿童作为健康对照。本研究获得医院医学伦理委员会批准及患儿家长知情同意。
1.2 诊断标准颞叶癫癎、特发性癫癎根据 1989 年国际抗癫癎联盟关于癫癎及癫癎综合征分类标准[15]进行诊断。颞叶癫癎的临床特点:如上腹部感觉异常、恐惧等先兆,口咽及运动自动症,或听觉、前庭、复杂视幻觉、梦样状态等先兆,继之出现全面性发作等;发作间期脑电图显示颞区导联癫癎样放电。特发性癫癎诊断标准:发作形式仅为全面强直阵挛发作;发作间期脑电图提示广泛性4~5 Hz多棘慢综合波或多棘波发放。
排除标准:头部影像学检查见结构性异常,单侧或双侧海马硬化及萎缩;伴影响智力的其他疾病或精神科疾病;除了服用抗癫癎药物以外,服用过其他影响智力的药物;神经系统体查异常。
1.3 量表测评由神经心理室医师完成。认知功能综合测评采用蒙特利尔认知测评量表(Montreal Cognitive Assessment Scale,MoCA)和儿童韦氏智力分量表(言语流畅性,木块图,数字广度)。焦虑抑郁情绪测评采用儿童社交焦虑量表(Social Anxiety Scale for Children,SASC),儿童抑郁障碍自评量表(Depression Self-rating Scale for Children,DSRSC)。
1.4 临床资料收集由癫癎专科医师采用问卷调查方式,进行资料收集。所有入选的患者填写调查表,内容主要包括人口学特征(性别、年龄、居住地、受教育程度)、患者的临床特征(发病年龄、病程、发作频率、抗癫癎用药种数等)、脑电图检查和神经影像学检查结果等。
1.5 统计学分析所有数据处理应用 SPSS 13.0软件完成。符合正态分布的计量资料以均数±标准差(x±s)表示,多组间比较采用单因素方差分析,组间比较采用LSD检验;不符合正态分布的计量资料采用中位数(四分位间距)[P50(P25,P75)]表示,多组间比较采用 Kruskal-Wallis H检验,组间比较采用Wilcoxon 秩和检验。计数资料采用百分率(%)表示,率的比较采用 χ2检验。相关性分析采用Pearson相关分析。检验水准为P<0.05。
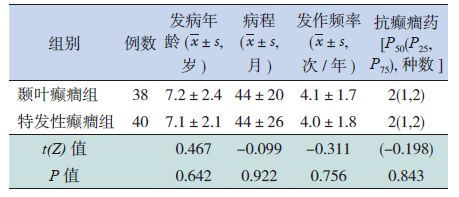
2 结果 2.1 受试者一般临床资料健康对照组、颞叶癫癎组、特发性癫癎组之间,患儿的年龄、性别、受教育程度差异无显著性(P>0.05),见表 1。颞叶癫癎组与特发性癫癎组患儿的发病年龄、病程、发作频率、抗癫癎用药种数比较,差异无统计学意义(P>0.05),见表 2。
| 表 1 颞叶癫癎组、特发性癫癎组和健康对照组的一般临床资料 |
| 表 2 颞叶癫癎与特发性癫癎患儿的临床特点比较 |
2.2 认知功能测评结果
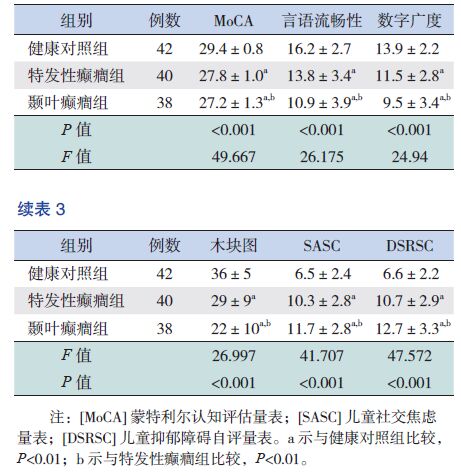
颞叶癫癎组与特发性癫癎组患儿在MoCA,言语流畅性,木块图,数字广度测试均低于健康对照组,差异有统计学意义(P<0.05),而颞叶癫癎组测试分低于特发性癫癎组(P<0.05)。见表 3。
| 表 3 健康对照组及颞叶癫癎、特发性癫癎组认知功能和焦虑抑郁情绪测评比较 |
2.3 焦虑抑郁情绪测评结果
颞叶癫癎组与特发性癫癎组患儿的SASC、DSRSC得分均高于健康对照组,差异有统计学意义(P<0.05);而颞叶癫癎组得分高于特发性癫癎组,差异有统计学意义(P<0.05)。见表 3。
2.4 癫癎患儿认知功能与焦虑抑郁情绪、发病年龄、病程、发作频率及抗癫癎用药种数的相关性分析颞叶癫癎组患儿的MoCA得分与SASC、DSRSC、发作频率得分均呈负相关(r=-0.571,-0.529,-0.545,P<0.001)。特发性癫癎组患儿的MoCA得分与SASC、DSRSC、发作频率得分呈负相关(r=-0.542,-0.487,-0.555,P<0.01)。颞叶癫癎患儿的MoCA得分与发病年龄、病程、抗癫癎用药种数及文化程度均无相关性(r=0.23,0.131,0.214,-0.34,P>0.05)。特发性癫癎患儿的MoCA得分与发病年龄、病程、抗癫癎用药种数及文化程度亦无相关(r=0.154,0.212,0.32,-0.25,P>0.05)。
3 讨论颞叶皮层具有复杂而重要的认知功能,一方面,颞叶癫癎与海马等边缘系统的病变关系密切,而海马参与了记忆、学习等认知功能的形成;另一方面,癫癎发作、发作间期癎样放电、抗癫癎药物、手术治疗等都可能对患者认知功能造成不利的影响。弥散张量成像研究发现,命名和言语流畅性功能主要由额颞叶纤维组成的网络负责,工作记忆主要由额顶叶和内侧颞叶网络负责正常运行。而在颞叶癫癎中,这些认知网络均因癫癎发作或手术干预而遭到干扰破坏[16]。有研究显示,70%的颞叶癫癎患者存在不同程度的记忆障碍,30%的颞叶癫癎患者智力低下[17]。本研究认知功能测评结果证实,儿童颞叶癫癎患者也存在明显认知功能受损,与Harden[17]研究相符。MoCA 量表评估的是整体认知功能,言语流程性、木块图、数字广度各项分测试则主要评估与额颞叶功能相关的言语、执行及记忆功能。本研究结果显示,颞叶癫癎患儿的整体智能及言语、记忆、执行功能较健康儿童明显下降,提示额颞叶认知网络普遍受损。本研究结果亦提示特发性癫癎患儿的认知功能普遍轻度下降,这与既往研究结果存在差异。既往研究证实成人及儿童特发性癫癎患者均存在言语流畅性、逻辑推理、语言性工作记忆、注意力不足等认知缺陷[8, 18-19],但非语言性记忆能力未受到明显影响[19]。本研究中,特发性癫癎组患儿的言语流畅性、语言性记忆(数字广度)及非语言性记忆(木块图)得分均低于健康对照组患儿,这可能与不同研究所选取的受试者人种及病程长短的不同有密切关系。因此还需要多中心大样本的研究进一步探索。再者,颞叶癫癎患儿认知功能下降比特发性癫癎患儿更严重,可能与主要负责学习、记忆等认知功能的内侧颞叶海马的受损密切相关,以往研究也证实了这一点[20]。
而且,本研究结果提示颞叶癫癎患儿存在明显焦虑抑郁情绪障碍,这与其他研究结果相似[21]。研究发现癫癎与抑郁焦虑情绪障碍存在共同的神经生物基础,即下丘脑-垂体-肾上腺轴的超兴奋性以及以五羟色胺与去甲肾上腺素为主的神经递质系统的紊乱。五羟色胺受体密度在颞叶和前额叶最高,临床及动物实验均证实大脑皮层 γ-氨基丁酸(gamma aminobutyric acid,GABA)体功能依赖性的大幅度降低是颞叶癫癎脑功能改变的普遍特征[22-24]。因此,我们认为颞叶癫癎患儿大脑皮层的GABA 神经递质功能受损引发患儿情绪调控紊乱,出现抑郁焦虑等负面情绪。本研究结果显示特发性癫癎患儿也伴有一定程度焦虑抑郁,目前国内相关报道较少。影像学研究从宏观角度发现,与健康对照组比较,颞叶癫癎患者的海马体积缩小,特发性癫癎患者杏仁核体积缩小[25],这可能是两组癫癎患者焦虑抑郁共患病的解剖基础。同时,本研究发现,颞叶癫癎患儿焦虑抑郁情绪障碍比特发性癫癎患儿更明显,这与既往研究结果相似[26]。颞叶新皮层以及内侧结构,如海马、杏仁核等是认知和情绪调节的关键部位,而颞叶癫癎患者的癎样放电起源和传播均集中在颞区,而特发性癫癎癎样放电多无固定部位。临床研究也证实颞叶癫癎的认知及焦虑抑郁障碍程度均重于特发性癫癎[20, 26]。
本研究相关性分析提示,颞叶癫癎患儿MoCA得分与SASC、DSRSC得分呈负相关,即颞叶癫癎患儿的焦虑抑郁情绪可以加重认知功能障碍。既往有研究针对颞叶癫癎患者的焦虑抑郁症状进行抗焦虑抑郁的认知和药物治疗,可以提高患者的生活质量并减少癫癎发作,这从反面证实了焦虑抑郁与认知功能呈负相相关性[16, 27]。并且,本研究发现,颞叶癫癎组与特发性癫癎组患儿MoCA得分与发作频率呈负相关,这也与既往研究结果一致[11],但患儿MoCA得分与发病年龄、文化程度、抗癫癎药物数量未发现相关性,这与既往研究[9]不同,这可能与我们样本量较小有关,未来还需要进行大样本多中心研究。再者,我们未对患儿癎样放电频率对认知功能的影响进行研究,这在今后的研究中需要进一步分析。
综上所述,我们首次在国内评估了颞叶癫癎及特发性癫癎患儿的认知功能及合并焦虑抑郁情绪状况,证实这两组患儿均存在认知功能下降及合并较高水平的焦虑抑郁情绪,尤其在颞叶癫癎患儿表现最为明显。同时发现两组患儿合并的焦虑抑郁情绪可以加重认知功能损害。因此,对于癫癎患儿及早进行认知功能评估和焦虑抑郁情绪筛查,并进行相关干预治疗是非常必要的,这对改善癫癎患儿的生活质量及预后,具有重要的临床意义。
| [1] | Samarasekera SR, Helmstaedter C, Reuber M. Cognitive impairment in adults with epilepsy: The relationship between subjective and objective assessments of cognition[J]. Epilepsy Behav,2015, 52 (A) :9–13 . |
| [2] | Kanner AM, Soto A, Gross-Kanner H. Prevalence and clinical characteristics of postictal psychiatric symptoms in partial epilepsy[J]. Neurology,2004, 62 (5) :708–713 . |
| [3] | Giovagnoli AR, Parente A, Tarallo A, et al. Self-rated and assessed cognitive functions in epilepsy: impact on quality of life[J]. Epilepsy Res,2014, 108 (8) :1461–1468 . |
| [4] | Cascino GD. When drugs and surgery don't work[J]. Epilepsia,2008, 49 (9) :79–84 . |
| [5] | Baxendale S, Heaney D, Thompson PJ, et al. Cognitive consequences of childhood-onset temporal lobe epilepsy across the adult lifespan[J]. Neurology,2010, 75 (8) :705–711 . |
| [6] | Elger CE, Helmstaedter C, Kurthen M. Chronic epilepsy and cognition[J]. Lancet Neurol,2004, 3 (11) :663–672 . |
| [7] | Marques CM, Caboclo LO, da Silva TI, et al. Cognitive decline in temporal lobe epilepsy due to unilateral hippocampal sclerosis[J]. Epilepsy Behav,2007, 10 (3) :477–485 . |
| [8] | Chowdhury FA, Elwes RD, Koutroumanidis M, et al. Impaired cognitive function in idiopathic generalized epilepsy and unaffected family members: an epilepsy endophenotype[J]. Epilepsia,2014, 55 (6) :835–840 . |
| [9] | Irwin LG, Fortune DG. Risk factors for psychosis secondary to temporal lobe epilepsy: a systematic review[J]. J Neuropsychiatry Clin Neurosci,2014, 26 (1) :5–23 . |
| [10] | Giovagnoli AR, Franceschetti S, Reati F, et al. Theory of mind in frontal and temporal lobe epilepsy: cognitive and neural aspects[J]. Epilepsia,2011, 52 (11) :1995–2002 . |
| [11] | Dodrill CB. Neuropsychological effects of seizures[J]. Epilepsy Behav,2004, 5 (1) :21–24 . |
| [12] | Drane DL, Ojemann JG, Kim MS, et al. Interictal epileptiform discharge effects on neuropsychological assessment and epilepsy surgical planning[J]. Epilepsy Behav,2016, 56 :131–138 . |
| [13] | Mula M, Trimble MR. Antiepileptic drug-induced cognitive adverse effects: potential mechanisms and contributing factors[J]. CNS Drugs,2009, 23 (2) :121–137 . |
| [14] | dos Santos EB, Tudesco Ide S, Caboclo LO, et al. Low educational level effects on the performance of healthy adults on a Neuropsychological Protocol suggested by the Commission on Neuropsychology of the Liga Brasileira de Epilepsia[J]. Arq Neuropsiquiatr,2011, 69 (5) :778–784 . |
| [15] | Anonymous. Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy[J]. Epilepsia, 1989, 30(4): 389-399. |
| [16] | Orjuela-Rojas JM, Martínez-Juárez IE, Ruiz-Chow A, et al. Treatment of depression in patients with temporal lobe epilepsy: A pilot study of cognitive behavioral therapy vs. selective serotonin reuptake inhibitors[J]. Epilepsy Behav, 2015, 51: 176- 181. |
| [17] | Harden CL. New evidence supports cognitive decline in temporal lobe epilepsy[J]. Epilepsy Curr,2007, 7 (1) :12–14 . |
| [18] | Henkin Y, Sadeh M, Kivity S, et al. Cognitive function in idiopathic generalized epilepsy of childhood[J]. Dev Med Child Neurol,2005, 47 (2) :126–132 . |
| [19] | Realmuto S, Zummo L, Cerami C, et al. Social cognition dysfunctions in patients with epilepsy: Evidence from patients with temporal lobe and idiopathic generalized epilepsies[J]. Epilepsy Behav,2015, 47 :98–103 . |
| [20] | Hitiris N, Mohanraj R, Norrie J, et al. Predictors of pharmacoresistant epilepsy[J]. Epilepsy Res,2007, 75 (2-3) :192–196 . |
| [21] | Rocha L, Alonso-Vanegas M, Martinez-Juarez IE, et al. GABAergic alterations in neocortex of patients with pharmacoresistant temporal lobe epilepsy can explain the comorbidity of anxiety and depression: the potential impact of clinical factors[J]. Front Cell Neurosci,2014, 8 :442. |
| [22] | Leroy C, Poisbeau P, Keller AF, et al. Pharmacological plasticity of GABA(A) receptors at dentate gyrus synapses in a rat model of temporal lobe epilepsy[J]. J Physiol,2004, 557 (2) :473–487 . |
| [23] | Ragozzino D, Palma E, Di Angelantonio S, et al. Rundown of GABA type A receptors is a dysfunction associated with human drug-resistant mesial temporal lobe epilepsy[J]. Proc Natl Acad Sci U S A,2005, 102 (42) :15219–15223 . |
| [24] | Goldberg H, Weinstock A, Bergsland N, et al. MRI segmentation analysis in temporal lobe and idiopathic generalized epilepsy[J]. BMC Neurol,2014, 14 (1) :1–20 . |
| [25] | Ertekin BA, Kulaksizoglu IB, Ertekin E, et al. A comparative study of obsessive-compulsive disorder and other psychiatric comorbidities in patients with temporal lobe epilepsy and idiopathic generalized epilepsy[J]. Epilepsy Behav,2009, 14 (4) :634–639 . |
| [26] | Crail-Melendez D, Herrera-Melo A, Martinez-Juarez IE, et al. Cognitive-behavioral therapy for depression in patients with temporal lobe epilepsy: a pilot study[J]. Epilepsy Behav,2012, 23 (1) :52–56 . |
 2016, Vol. 18
2016, Vol. 18