The goal of nutrition of the preterm infant is to meet the growth rate of the healthy fetus of the same gestational age[1]. It also is important to produce the same body composition of the healthy fetus in terms of organ growth, tissue components, and cell number and structure. Failure to provide the necessary amounts of all of the essential nutrients has produced not only growth failure[2], but also increased vulnerability to infectious diseases arising from suboptimal immune defenses[3], increased respiratory distress from lung injury due to muscle weakness[4], impaired tissue repair in response to injuries (such as oxygen toxicity and barotrauma in the lung, inflammation in the gut, and wound healing), and general underdevelopment of all other organs such as intestine[5-6], skeletal muscle[7], and brain[8-9].
As documented by many studies over many years, neuronal underdevelopment may be the most serious adverse consequence of under nutrition of preterm infants, which clearly leads to later life cognitive deficiencies[10-13]. The most severe adverse neurodevelopmental outcomes occur in preterm infants who had IUGR and also suffered postnatal under nutrition. While it remains uncertain whether improved neuronal development, brain growth, and cognitive outcomes are the direct result of improved nutrition or whether healthier infants can be fed more[14], studies clearly are documenting the association of improved neurodevelopmental outcome with improved nutrition and growth of preterm infants[15-17].
Despite such needs, it remains a challenge to consistently provide adequate nutrition to preterm infants, particularly right after birth when nutritional support is largely dependent on intravenous nutrition and often is complicated by glucose and lipid intolerance[18-19]. During this period, large nutritional deficits, in particular protein deficits, can rapidly accumulate in preterm infants. In such infants, protein stores can decline by as much as 1.5%/day, in contrast to the normally growing fetus that has a positive net protein balance of ~2%/day[20]. Many studies from around the world have provided consistent evidence that early amino acid intake, at least 3g/kg/day, before 5 days of life, can reduce protein breakdown and deficits and improve protein balance and growth outcomes in preterm, very-low-birth-weight infants[21-27]. This is one of the most consistent observations among controlled research trials and observational studies in neonatal medicine[19].
Nevertheless, growth failure is almost universal among preterm infants[28]. 80% of very low-birth weight preterm infants involved in studies conducted by the NIH Neonatal Research Network from 2008 to 2010 had documented growth failure[29-30], and among infants within the Vermont Oxford Hospital Network, at least 50% were growth-restricted at the time of discharge from the NICU[2]. Such growth failure is, however, preventable, and so is the neurodevelopmental impairment that comes from insufficient nutrition. Protein deficiency is critical, in this regard, as protein is the nutritional component most essential for development of neurological function[31], but overall nutritional quantity and quality also are fundamental for normal growth and development, including neurodevelopmental outcomes. Growth velocities during the NICU hospitalization period for preterm infants exert a significant, and possibly independent, effect on neurodevelopmental and anthropometric outcomes[13]. As shown by Lucas and colleagues[32], 4 weeks of enriched formula with greater amounts of energy as well as protein fed to preterm infants improved growth and neurodevelopmental outcomes at 18 months corrected gestational age when compared to preterm infants fed lower amounts of energy and protein in standard term formulas. At 7.5 years of age, the infants fed the enriched preterm formula demonstrated higher cognitive functions[33], and even at age 16, these infants had larger brain size and larger volumes of the caudate nucleus measured using MRI F:\Documents\Neonatal Nutrition, for Bo Sun, Chinese Journal of Comtemporary Pediatrics\Nutritional_support_for_extremely_low-birth_weight_infants_abandoning_cat.html - 134 and higher intelligence quotient (IQ) scores[16, 34]. Regression analysis of infants from several studies have shown a significant positive correlation between both cumulative energy and protein intakes and greater head circumference at 36 weeks postmenstrual age[35], F:\Documents\Neonatal Nutrition, for Bo Sun, Chinese Journal of Comtemporary Pediatrics\Nutritional_support_for_extremely_low-birth_weight_infants_abandoning_cat.html - 136and better mental and psychomotor developmental index (MDI and PDI) scores at 3 months' corrected age[36]. Furthermore, a recent retrospective study showed that the MDI of ELBW infants at age 1.5 years increased by 8.2 and 4.6 points, respectively, for every extra gram of protein/kg per day or every extra 10 kcal/kg per day they received during the first week of life[37]. This early postnatal period appeared to be critical, as nutritional intakes during the next few weeks of life did not show correlations with neurocognitive outcome. While continued nutritional support during the NICU period, as well as after discharge, is fundamental, clearly, the first few days after birth are most important for achieving positive protein and energy balance and for promoting improved growth and development.
There is every reason, therefore, to optimize nutrition of the preterm infant, in terms of total energy and protein, but also in terms of individual components such as amino acids, specific carbohydrates and lipids, and even oxygen. If this is the case, how successful is current nutritional practice for preterm infants in terms of producing optimal nutrition and growth and development of preterm infants? The answer is, universally, not as well as it should. It is imperative, therefore, to reassess the rate of growth that should be achieved in preterm infants and the basic nutritional requirements needed to achieve this growth rate.
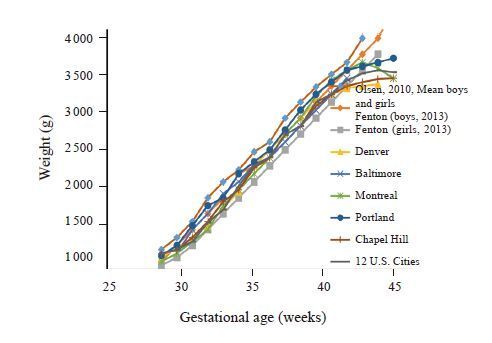
How fast should a preterm infant grow?Direct measurement of normal human fetal growth is not possible. Most commonly, therefore, fetal growth rates have been estimated from anthropometric measurements at the time of birth among “healthy”, normal appearing preterm infants of viable gestational age through to term. Using this approach, multiple studies have shown that the average fractional fetal weight gain from 24 to 38 weeks gestation is 17 g/kg/d[38-40] (Figure 1).
|
Figure 1 Birth weights by gestaional age [38-40] Regardless of the growth curve, normal human fetal growth rate is ~17 g/kg/day from 28-40 weeks. |
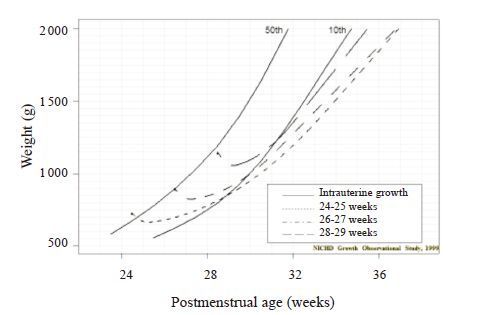
Most studies also show, however, that most preterm infants fail to grow well after birth, usually for many days. They also don’t keep up with intrauterine growth and thus end up growth restricted by term[41-42] (Figure 2). This problem has not improved over the past almost 70 years[43].
|
Figure 2 Postnal growth of VLBW infants vs. expected intrauterine growth [42] |
While there are many possible reasons why preterm infants do not grow after birth as well as the normal fetus of the same gestational age, including stress responses (e.g., increased secretion and plasma concentrations of catabolic hormones such catecholamines, glucagon, and cortisol, intermittent hypoxia, respiratory distress, mechanical ventilation, sepsis, inadequate body temperature maintenance, etc.), a principal cause is insufficient nutrition, as evidenced by cumulative deficits in energy and protein, even with more “aggressive” nutrition that provides closer to estimated nutrient requirements[44] (Figure 3) .
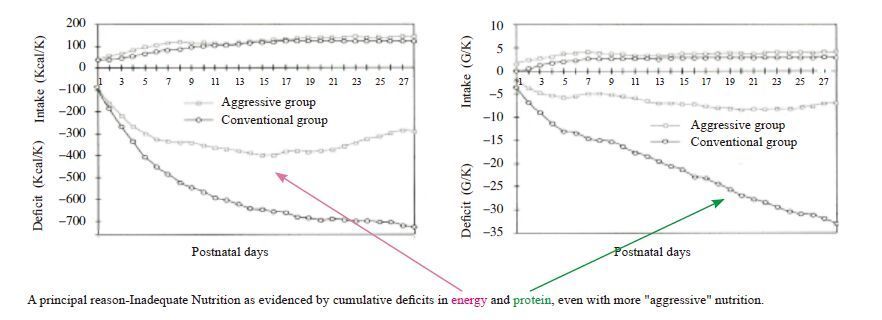
|
Figure 3 Energy and protein intake and cumulative deficits in the first 28 postnatal days [44] |
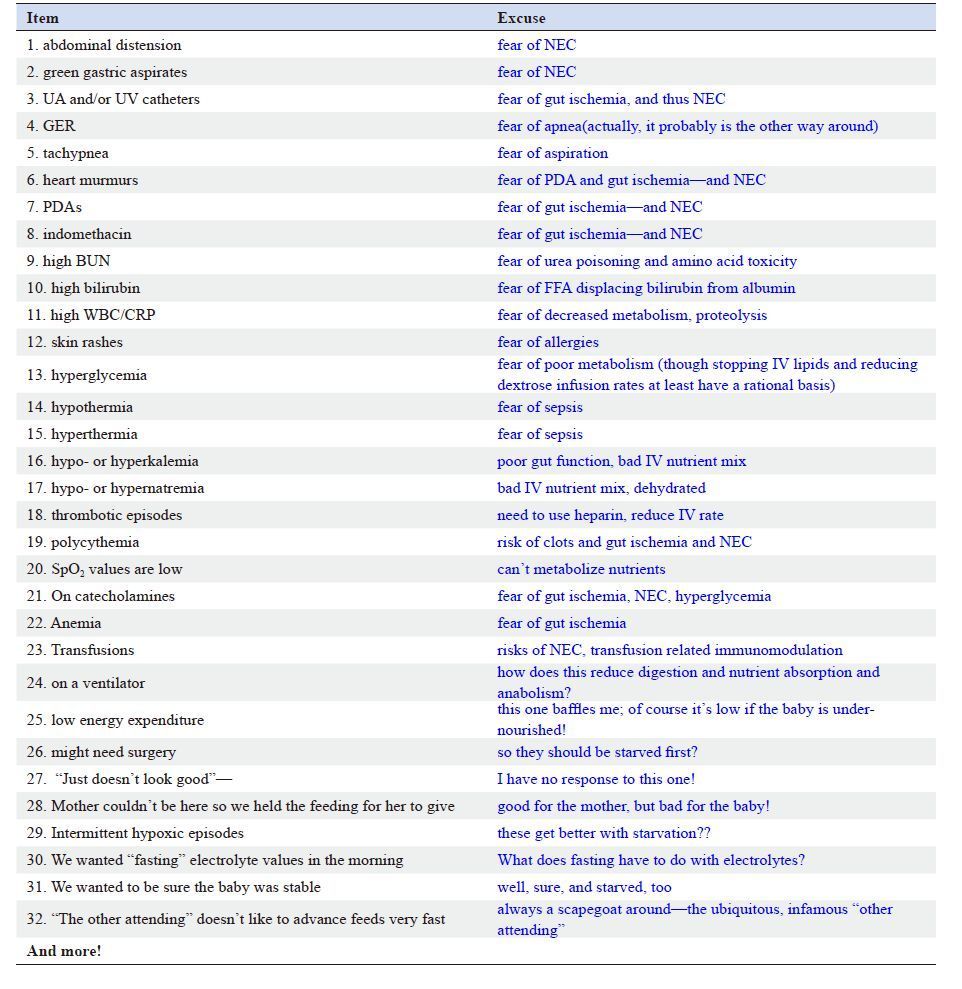
Why are preterm VLBW infants not fed enough to grow as well as the fetus? There are several practical and common reasons. First, most preterm infants have a delayed start to receiving nutrients, e.g., low rates of or even no IV amino acids on the first day of life (to sometimes several days after birth). Second, enteral feedings often are withheld, sometimes for days. Third, most infants receive very slow advances of nutrient supply, e.g., IV amino acid infusion rates of <3 g/kg/d. Fourth, there are usually rather slow advances of IV amino acids and lipids after starting, and slow advances of enteral feeds. Fifth, most infants receive dilute nutritional mixes, e.g., unfortified breast milk (mother’s own or banked), and insufficient amounts of essential amino acids in TPN mixes. There also are many excuses offered as “reasons” for withholding, slowing down, or stopping feedings, practically none of which has a rational basis from controlled trials. For the most part, it is simply assumed that infants with any kind of physiological instability cannot tolerate normal amounts of nutrition, either parenteral or enteral (Table 1). As a result, preterm infants commonly are under nourished from birth and often for days to sometimes weeks, and all of the practical reasons and excuses, justified or not, reduce nutrient intake, which leads to growth failure and impaired neurodevelopment. It is not unexpected, therefore, that growth, which for the fetal period of human development requires constant supplies of nutrients, does not occur or even decreases relative to that of the normally nourished and normally growing human fetus of the same gestational age.
| Table 1 “Excuses” for withholding, slowing down, or stopping feeding of preterm infants, with comments about why this might be done and why it might not be rational |
Nutrient requirements for preterm infants
If we are going to feed preterm infants to grow as well as the normal, healthy human fetus, we should consider the normal fetal nutrient and co-factor requirements and at least provide those. There may be additional adjustments for conditions that arise after birth, but it is unreasonable to consider nutrition sufficient if it provides less than what the normal fetus receives. What nutrients and co-factors, therefore, should we provide to the preterm infant at the same gestational age of the fetus in utero?
OxygenFirst, while not actually a nutrient, oxygen is necessary for the metabolism of all nutrients and particularly for producing growth. Numerous studies have shown that hypoxia and oxygen consumption rates less than normal restrict growth. The normal fetus exposed to hypoxia responds by increasing erythropoietin, producing more red blood cells, thereby increasing its blood oxygen content to compensate for the reduction in molecular oxygen supply[45]. Also, many studies have shown that preterm infants who receive blood transfusions for severe anemia not only increase their blood oxygen content but also improve their growth rate. In one study, for example, preterm infants did not gain weight as well when their Hb concentration was ≤ 8.5 g/dL (Hct ≤ 25%), but their weight gain improved after transfusion with red blood cells to a blood Hb concentration of ≥ 11.4 g/dL (Hct ≥ 34%). The more severe the anemia (lower the blood oxygen content), therefore, the more likely that growth failure will develop[46]. Commonly, however, current practice usually reduces blood oxygen content by allowing preterm infants to be more anemic (hematocrits in lower 20 s) to limit transfusion risks (transfusion-related inflammatory disorders, e.g., NEC, strokes, lung injury) and to be maintained at lower PaO2 values to prevent oxygen toxicity (e.g., ROP, BPD). Furthermore, preterm infants lose, by adult standards, enormous amounts of blood for all sorts of biochemical and hematological measurements. Unfortunately, we have little capacity to measure the clinical effect of low O2 supply on nutrient metabolism (energy + protein), protein accretion, and neuronal growth and development, limiting our ability to assess the impact, in any given infant, of different low blood oxygen contents on producing growth restriction. Our goal, therefore, should be to determine optimal nutrient and anabolic hormone supplies to promote growth, especially of neurons/dendrites, at lower blood O2 levels. We also might improve our administration of erythropoietin (give more, or develop more effective protocols), provide safer transfusions, use delayed cord clamping and cord stripping routinely, reduce blood sampling, microsize blood sample assays, and provide more and better noninvasive monitoring.
GlucoseAdequate glucose also must be provided to ensure appropriate growth rates. Glucose is essential for normal metabolism in all cells, but as the principal energy substrate of the normal fetus and thus the preterm infant, glucose is necessary to support growth, particularly protein synthesis from amino acids and net protein balance. In fetal studies, for example, reduction in glucose supply and plasma glucose concentrations lead to growth failure[47]. This is a relatively uncommon problem in preterm infants, however, as most of the time, they are infused with more glucose than they can use, resulting in hyperglycemia. Normal neonatal glucose production and utilization rates have been estimated from animal studies[48] and measured in infants with stable isotope techniques[49-50]. Preterm infants at ~28 weeks gestation maintain hepatic glucose production rates of 2-3 mg/min/kg which are not easily suppressed, either by glucose or insulin, as they are in normal children[51]. Glucose utilization rates can be as high as 7-9 mg/min/kg, however, largely for the brain, but also for the heart. Term infants also produce ~2-3 mg/min/kg of glucose, but their glucose utilization rates are lower at ~3-5 mg/min/kg (still largely for the brain). The decrease in weight-specific glucose utilization rates from very preterm to term gestational age is the result of increasing body proportions of organs, such as bone, muscle, lung, and the gastrointestinal tract, that do not use glucose as much as the brain and heart. Regardless of the utilization or production rates, preterm infant plasma glucose concentrations should be in the same range as normal human fetuses of the same gestational age, i.e., between 3.0 mmol/L and 6 mmol/L[52]. This is the same range achieved by normal, healthy term newborn infants after the first 12-24 hours from birth following the normal nadir in glucose concentration from 1-3 hours of age[53].
LipidsNormal human fetal development involves considerable fat deposition in adipose tissue, primarily peripherally in subcutaneous regions of the body. Normal human fetuses produce about 15 g/kg body weight as fat by term. Early in the third trimester (24-28 weeks), however, there is little lipid uptake, oxidation, or accretion as fat by the fetus. Lipid supply and fat accretion increase progressively over the third trimester. It remains uncertain how much lipid is oxidized in the human fetus, but likely not very much, as the concentrations of carnitine palmitoyl transferase, the enzyme that transports long chain fatty acids into the mitochondria for lipid oxidation, are quite low, and glucose and amino acid supplies are more than sufficient to meet most energy requirements. After birth, however, even in very preterm infants and even quite soon after birth, supplemental carnitine (e.g., in mother’s milk) promotes lipid oxidation.
Extremely preterm infants often are slow to clear plasma of lipid due to maturational deficiency of lipases (inversely related to GA), low levels of carnitine palmitoyltransferase (CPT), and chronically reduced carnitine intakes >2 weeks after birth with prolonged IV nutrition[54]. There also is competition of free fatty acids for oxidation due to early postnatal hyperglycemia. As a result of such conditions, many recommend limiting lipid intake to 0.5-1 g/kg/day in presence of other disorders that limit fatty acid oxidation, such as sepsis, severe lung disease, surgical stress, steroid use, persistent hyperglycemia, and cholestasis. This practice is poorly justified, however, with little evidence for mechanisms involved and how to manipulate them, other than added carnitine to promote better fatty acid oxidation.
The quality of fatty acids and triglycerides and other lipid products also is important[55]. Little information exists, however, about the actual mix of such substances in fetal plasma that might provide guidance for the optimal mix of plasma lipid substances after birth. A major concern, however, is how to provide sufficient long chain polyunsaturated essential fatty acids that are fundamental components of the developing nervous system[56]. Normal fetal white adipose tissue accumulation in the 3rd trimester of the essential omega 3 long chain polyunsaturated fatty acids (PUFAs), particularly docosahexaenoic acid (DHA, 22:6n-3) ranges from ~45 to ~70 mg/day. According to current practice, at 3.7g fat/dL human milk with 0.2-0.4% fatty acids as 22:6n-3, a 1 kg preterm infant fed at full enteral feeds of 180 mL/day would get only 13-25 mg 22:6n-3/day, clearly far below normal in utero accretion rates. As shown by Lapillone and colleagues, therefore, postnatal DHA deficiency is an inevitable consequence of current recommendations and practices for feeding milk, milk supplements, and formulas in preterm infants[57]. In terms of potential benefits of providing greater amounts of DHA, one recent study has shown that higher DHA and lower linoleic acid levels in the first few weeks of life are associated with decreased intraventricular hemorrhage, improved microstructural brain development, and improved outcomes in preterm infants[58]. Several other studies also have shown that preterm infants fed increased DHA have higher visual acuity, particularly at 2 and 4 months and improved Bayley mental development and MacArthur Communicative inventories at 12 months[59], but longer term studies do not show a clear benefit. Thus, the current diet for preterm infants is deficient in at least this one essential fatty acid, but the long term significance of this deficiency is not known or how these infants would develop if fed to sufficiency.
EnergyIn terms of total energy (primarily from carbohydrates and lipids), there is a persistent but unsubstantiated concern that higher intake rates of amino acids and protein won’t produce more growth unless higher calorie intakes also are provided. This concern is based on the unjustified fixation on a continuous 22 kcal/g protein ratio. Actually, excessive caloric intake simply increases body fat content relative to lean mass. Above 80-90 kcal/kg/day non-protein caloric intake, there is no further increase in net protein balance for any further increase in energy intake. Protein gain primarily depends on protein intake, regardless of the energy intake[60]. This has been corroborated by elegant nutritional balance studies by Kashyap and collegues[61] (Figure 4), who showed that while greater protein intakes would increase weight, length, and head circumference, additional lipid with the greater protein intakes only increased weight and subcutaneous fat.
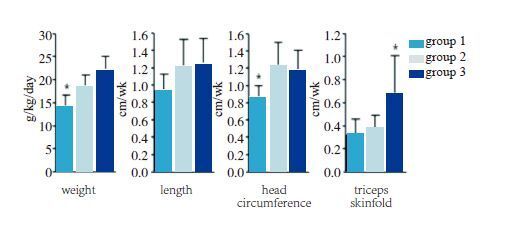
|
Figure 4
Growth rates with varying protein and
energy intakes [61]
Preterm infants, birth weight 900-1 750 g: Group 1, 2.24 g/kg/day and 115 kcal/kg/day; Group 2, 3.6 g/kg/day and 115 kcal/kg/day; Group 3, 3.5 g/kg/day and 149 kcal/kg/day. *P<0.05: weight in group 1 less than in group 2 and group 3; head circumference in group 1 less than in group 2 and group 3; triceps skinfold greater in group 3 than in group 1 and group 2. |
Despite such evidence, recent observations of actual intake of nutrients in clinical neonatal practice has been weighted to energy, both carbohydrates and lipids, and not to protein, leading to problems of carbohydrate and lipid excess and protein insufficiency[62]. Such a diet mix leads to fatter, shorter, less muscular infants, and perhaps to longer term neurological deficits. Unfortunately, this problem is most common among the smallest, most preterm infants who are fed far more energy and much less protein than needed to meet appropriate growth rate and body composition[63].
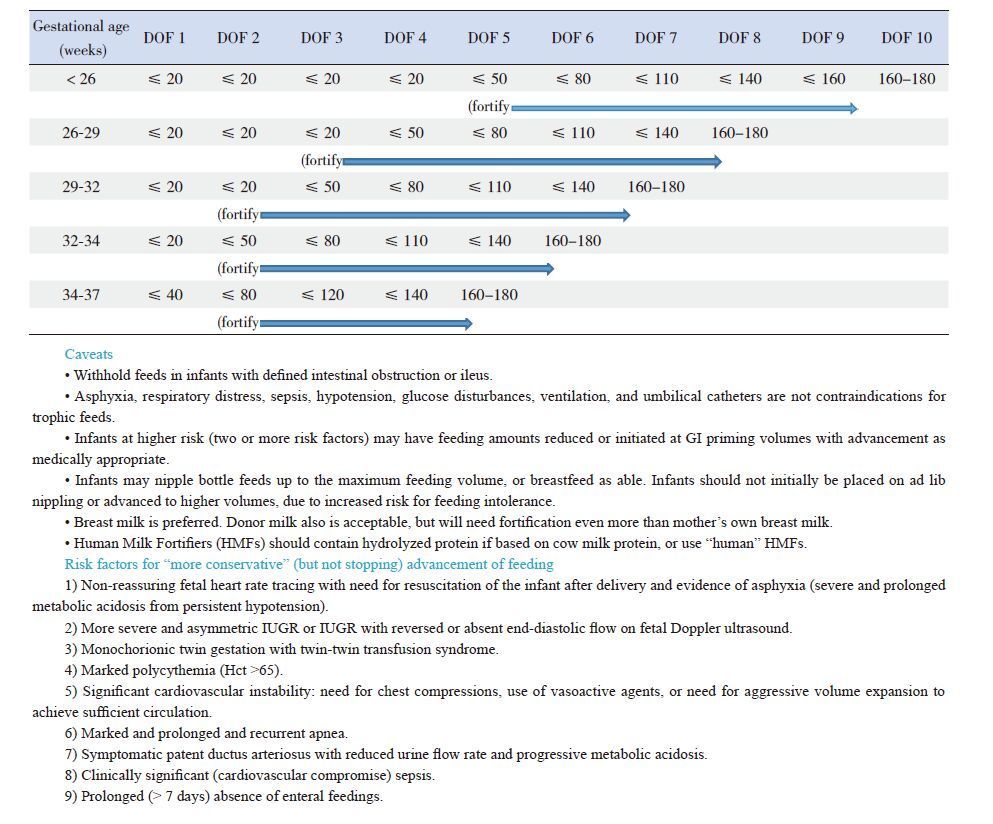
Amino acids and proteinThe most important requirement for growth is protein intake, as amino acids intravenously and as intact to partially or fully hydrolyzed protein enterally. Lean body mass, which consists mostly of protein, accounts for 90% of growth in the 3rd trimester of gestation. Without sufficient protein, cellular replication, hypertrophy, and development cannot take place as optimally as they should, and insufficient protein intake will adversely affect proliferation and growth of all cells in all organs (Table 2) [28].
| Table 2 Feeding guidelines: intake amounts (mL/kg/day), by day of feeding (DOF) |
The reason for this need for protein is that protein synthesis and accretion rates are very high in the fetus (net protein balance of ~2%/day) and therefore the preterm infant of the same gestational age, requiring large amino acid uptake rates. Fetal animal growth data, when scaled to human fetal growth rate, predict fetal amino acid requirements ≈ 3.6-4.8 g/kg/day at ~24-28 weeks of human gestation. This animal data has been corroborated for human fetuses by the Factorial Method[64], which predicts that human fetal amino acid requirements ≈ 4 g/kg/day at 24-28 weeks of gestation. It is important to note, however, that fractional protein synthesis rates decrease with gestational age and development. Thus, between 30 and 37 weeks, the protein requirement for growth decreases to ~ 2-3 g/kg/day and by term, protein requirements decrease to those of the normal breast fed infant, or ~ 1.5-2 g/kg/day. Among many studies in preterm infants, net protein balance as measured by net nitrogen retention is directly and linearly related to protein intake[19, 65]. Specific studies demonstrate the same linear relationship between IV amino acid intake and protein balance through 3 to 4 g/kg/day[22, 66].
Practical feeding guidelines: intravenous feedingThe goal of early intravenous feeding is to maintain normal cellular energy and amino acid supplies. Metabolic and thus nutritional requirements do not stop with birth, and the smaller the infant, the less body stores of nutrients are available to provide nutrients for metabolic needs. Intravenous feeding, therefore, is always indicated when normal metabolic and nutritional needs are not met by normal enteral feeding. Furthermore, the metabolic and nutrient requirements of the newborn preterm infant are equal to or greater than those of the fetus of the same gestational age. It is reasonable, therefore, to provide the preterm infant with at least the same nutrient supplies that the fetus of the same gestational age receives for nutrition. Although early intravenous nutrition appears to be a beneficial clinical strategy[67], the optimal amount of that support is not fully known[19], including the time after birth to start, the doses to use, and the rate of advancement[68]. The Current recommendations are that IV nutrition should be initiated within a few hours of birth[28].
Amino acidsAt least 2.0 g/kg/day of amino acids should be started within 2-3 hours of birth, and increased to 3.5-4.0 g/kg/day over the first 24-48 h for infants <30 weeks gestation (up to 4.0 g/kg/day for extremely low birth weight infants <27 weeks gestation).
Even when solutions are infused at these or yet higher rates, intakes of certain essential amino acids, particularly the branched-chain amino acids such as leucine and isoleucine[69], but also threonine and lysine[70], may not produce high enough plasma concentrations to promote the rate of cellular essential amino acid uptake needed to produce appropriate protein accretion. There has been some concern for excessively high plasma amino acid concentrations at infusion rates >3.0-4.0 g/kg/day, particularly in sick and physiologically unstable preterm infants[71]. Also, infants who experienced severe and chronic intrauterine growth restriction may have developed adaptations that actually limit amino acid synthesis into protein[72]. There is little evidence, however, that most preterm infants will have significant complications from the amino acid infusion rates noted as necessary to produce in utero protein accretion and growth rates. In fact, most studies have noted that current IV amino acid mixtures and infusion rates produce lower plasma concentrations than are needed for optimal amino acid metabolism and protein balance[22, 69-70]. The overall impact of such temporarily insufficient amounts of essential and total amino acids on growth and neurodevelopment of preterm VLBW infants is uncertain, but it is quite likely that even temporary shortfalls limit growth.
GlucoseGlucose (as 10% Dextrose in water) should be infused as soon as possible after birth to prevent hypoglycemia and at the highest rate tolerated without causing hyperglycemia.
Hypoglycemia, defined arbitrarily as a plasma glucose concentration <45 mg/dL in the 1st 2 days after birth and <50-60 mg/dL after 2 days of life, should be avoided as much as possible. While low glucose concentrations do occur even among preterm infants receiving IV dextrose infusions or TPN, there is no evidence that occasional transiently low values in the 40-50 mg/dL range are a problem.
Hyperglycemia, defined arbitrarily as a plasma glucose concentration >120 mg/dL at any time after birth, is a more common glucose metabolic disorder than hypoglycemia in preterm infants[73], largely the result of excessive dextrose infusion rates (>6-8 mg/kg/min) in the first hours to days after birth when stress reactive hormones that independently contribute to glucose production, such as norepinephrine and cortisol, are commonly present[74]. Hyperglycemia increases energy expenditure (glucose-to-fat synthesis is energy expensive), oxygen consumption (leading to hypoxia), carbon dioxide production (leading to tachypnea), fat deposition in excess of lean mass, and fatty infiltration of heart and liver. Hyperglycemia in preterm infants also is a potentially serious cause of cellular and even systemic inflammation from excess reactive oxygen species production[75-76]. Such effects may underlie the increased risk of retinopathy of prematurity noted among infants with hyperglycemia[77-79]. As noted in one recent study, at every time point in the first 12 hours after birth, hyperglycemia produces more unfavorable outcomes in infants with hypoxia-ischemia[80]. There also is the potential for sustained hyperglycemia in preterm infants to diminish longer term growth and even cognitive development, while also more positively diminishing body fat content. Down-regulation of the growth IGF-1/growth hormone axis may be responsible[81].
While hyperglycemia usually occurs in the first few days after birth, particularly among the most immature infants, it can be found at any time during IV nutritional support. In addition to the most common cause of hyperglycemia, which is excessive dextrose infusion, hyperglycemia also is the result of persistent hepatic glucose production[50] that is not down-regulated by increasing plasma concentrations of glucose or insulin[51]. Hyperglycemia also is caused by catecholamine treatment of low blood pressure, hydrocortisone treatment of low blood pressure and evolving chronic lung disease, and high IV lipid infusion rates.
Mixed hyper- and hypoglycemia occur quite commonly in very preterm, IUGR infants, but should be considered in any very preterm infant, as many of these infants experienced IUGR before their preterm birth. Hyperglycemia is common in such infants due to reduced pancreatic β-cell number and insulin production, hepatic insulin resistance and increased glucose production, decreased glucose disposal capacity because of their smaller brains and reduced muscle, and higher secretion rates and plasma concentrations of catecholamines and cortisol that limit insulin production and action. Hypoglycemia is common in such infants due to their greater head/brain to body/liver ratio and thus greater body weight-specific glucose utilization rate, increased peripheral tissue glucose uptake capacity from increased or at least maintained tissue glucose transporters, increased fractional insulin secretion and susceptibility to metabolic stimulation of insulin secretion, and good health and care that reduces catecholamine and cortisol secretion and concentrations and their negative impact on insulin secretion and action[82].
Prevention and treatment of hyperglycemia is important and is best accomplished by using lower IV dextrose infusion rates in the first place and lowering them, along with reducing lipid infusion rates and catecholamine and cortisol treatments and preventing hypoxic episodes. Use of insulin should be reserved for the most serious hyperglycemic conditions (plasma glucose concentrations >200-250 mg/dL and unresponsive to other approaches). There are many complications of using insulin to prevent or treat hyperglycemia. Insulin actually makes the baby fatter (including fatty infiltration of heart and liver). Insulin increases complications of excess allostatic load (reactive oxygen species production and inflammation). Insulin increases the risk of hypoglycemia[83]. Furthermore, although not studied yet in preterm infants, in children with cardiac disorders in intensive care, tight glucose control did not improve outcomes, but did result in more cases of hypoglycemia, and those children had poorer neurodevelopmental outcomes[84]. Infused insulin does not promote glucose uptake or utilization by the brain or enhance neuronal growth or dendritic development. Not the least, negative feedback mechanisms limit the effect of insulin to promote protein synthesis, net protein balance, and growth; insulin just does not work as a growth hormone to augment growth when given in excess. When insulin infusion was compared to reduced IV glucose infusion rate to treat hyperglycemia in 500-750 g infants[85-86], there were no differences in all age/weight groups for rates of death, sepsis, ROP, NEC, intracranial hemorrhage, chronic lung disease, days in the NICU, or rates of growth. It makes more sense to just limit the glucose infusion rate, and perhaps to increase IV amino acid infusion rates to 4 g/kg/day, which has been shown to reduce the number of episodes of hyperglycemia and the time-averaged plasma glucose concentration[87].
LipidsIntravenous lipid infusion should be started within 24 hours of birth and advanced from 2.0 to 3.0-3.5 g/kg/day as tolerated, usually over 2-3 days. Intravenous lipids provide essential carbon for oxidative metabolism and also provide essential fatty acids that promote membrane formation, particularly in the brain, but also in all cells in the body. Excess lipid infusion rates, however, commonly produce hypertriglyceridemia (arbitrarily defined as plasma triglyceride concentrations >150 mg/dL), which has been associated with adverse lipid deposition and subsequent inflammation in hepatocytes[62, 76], that may underlie the common problem of cholestatic jaundice and liver failure in infants who experience prolonged Intravenous nutrition[88]. ELBW infants often are slow to clear plasma of lipid due to maturational deficiency of lipases (inversely related to GA), low levels of carnitine palmitoyltransferase (CPT) and chronically reduced carnitine intakes >2 weeks after birth, and competition of FFA oxidation with carbon from hyperglycemia. In response, many recommend limiting lipid infusion rates to 0.5-1 g/kg/day in the first few days of life, particularly in presence of infection, severe lung disease, surgical stress, and steroid use. There is little rational justification for this practice, except when hypertriglyceridemia occurs in the presence of hyperglycemia, and to prevent cholestasis. Some have added carnitine to promote better fatty acid oxidation, but possible benefits only appear after total IV nutrition of more than 2 weeks[54].
It is clear, however, that postnatal deficiencies of essential fatty acids, particularly the long chain polyunsaturated fatty acid (LCPUFA) docosahexaenoic acid or DHA is an inevitable consequence of current recommendations and practices of intravenous lipid feeding, as well as for feeding milk, milk supplements, and formulas in preterm infants, leading to increasing cumulative DHA deficits. Unfortunately, to date, long term neurodevelopmental benefits of DHA or omega-3 LCPUFA supplementation are equivocal and shorter term improvements in BPD, ROP, NEC, sepsis, and death are uncertain.
Total energy: Intravenous nutrition should achieve a caloric intake of 50 kcal/kg/day on day 1 and 80 kcal/kg/day by day 3.
Amino acids: Intravenous amino acid infusion should be started right after birth, at least at 2.0 g/kg per day, and advanced to gestational age specific protein needs by day 2 to 3.
Between 24-30 weeks, initial intravenous amino acid infusion rates should range from 3.5 to 4.0 g/kg/day. Between 30 and 36 weeks, as fractional protein synthesis rates decline, intravenous amino acid infusion rates of 3.0 to 3.5 g/kg/day are appropriate. At term, amino acid requirements decrease to those of the normal breast fed infant, or 1.5-2.0 g/kg/day. While some have questioned whether the early, high amino acid infusion rate in very preterm infants might lead to potential adverse outcomes, previous observational and randomized controlled trials have not found smaller head circumferences in response to higher amino acid intakes[23-27, 89], and one recent randomized controlled trial showed that higher amino acid intakes resulted in greater head circumferences[27]. Even small differences in head circumference are unlikely to demonstrate developmentally important differences in brain volumes when early, higher amino acid infusion rates are used, and the studies addressing this issue actually have shown that the head circumferences measured are just above or just below the 50th percentile for head circumference at 40 weeks corrected gestational age[90], representing improved head circumference percentiles from those measured at birth[19]. Furthermore, even at 3.5 g/kg/day (if actually achieved), there may be inadequate essential amino acids to produce plasma concentrations sufficient to achieve appropriate cellular amino acid uptake rates and, thus, rates of protein synthesis[69-70, 89, 91].
Practical feeding guidelines: transition from intravenous to enteral feedingDuring the transition from parenteral to enteral nutrition, a shortfall of nutrient intakes may lead to insufficient protein and energy intakes[92]. This problem arises when parenteral nutrition is diminished or terminated early in order to limit the use of indwelling catheters because of the associated risk of infection (central line associated blood stream infection) and to limit total water intake and its potential to produce metabolic (dilutional acidosis, hyponatremia) and cardiopulmonary (PDAs) disorders[93]. As noted by Hay and Ziegler[28], practical solutions to this problem include temporarily increasing the total and especially the essential amino acid concentrations in parenteral nutrition solutions, continuing parenteral nutrition for longer periods, and advancing enteral nutrition and milk fortification sooner and at faster rates[94]. Fortification also should be started before full enteral feeding rates are achieved, perhaps at 50 mL/kg/day[92]. A recent study in 100 VLBW infants randomized to early fortification of enteral feeding at a feeding volume of 20 mL/kg/day or delayed fortification at a feeding volume of 100 mL/kg/day (all infants were fed according to a standardized enteral and parenteral feeding protocol; fortification was with Mead Johnson Nutrition acidified human milk fortifier) reported that the infants randomized to early fortification had a higher median daily protein intake at 1, 2 and 3 weeks of age as well as a higher cumulative protein intake in the first 4 weeks of life. There was no difference in days to reach full feeding volumes, episodes of feeding intolerance or necrotizing enterocolitis[95].
Practical feeding guidelines: enteral feedingThere are many adverse consequences of intravenous feeding only with no enteral nutrition. Problems of exclusive intravenous feeding include increased rates of sepsis (both due to suppressed immunity, reduced milk-associated immune function, and abnormal gut microbiome, as well as central line associated bacterial infections or CLABSI), gut mucosal atrophy and lack of production of gut-derived trophic hormones, increased adipose tissue and fatty infiltration of the heart and liver, systemic inflammatory response syndrome, reduced mucosal IgA from unstimulated and under developed Payer’s patches, and increased adhesion molecules and polymorphonuclear cell attraction. Animal models such as the neonatal piglet have shown reduced accretion of lean mass and bone mineral content as well as increased fat mass when neonatal piglets were fed TPN exclusively vs. enteral nutrition over a 16-day period[74]. Studies in adults[96-98] and older infants and children including term infants[99] have shown greater rates of various morbidities and even mortality when enteral nutrition is withheld and intravenous nutrition is used exclusively. Some of these problems most likely are the result of excessive glucose infusion with hyperglycemia, even when insulin is added as part of “tight glucose control” to maintain normal glycemia[100]. For older infants, as in children and adults, delaying intravenous nutrition while optimizing very early enteral nutrition appears to be safer, with fewer cases of infection and fewer days to discharge.99 Starting enteral nutrition as soon as possible, therefore, is beneficial, particularly when combined with appropriate early intravenous nutrition[101-102].
Of particular note is the improvement in gut development and growth using “trophic” or “gut priming” enteral feeds. Benefits include improved mucosal growth and development that clearly depend on enteral (not parenteral) nutrients, improved feeding tolerance and growth, less need for phototherapy, decreased cholestasis, decreased osteopenia, improved gastrointestinal trophic hormone surges, and improved GI motility, all with no increase in complications (e.g., NEC, particularly with colostrum and milk)[103-104]. It also is important to note that enteral feeding increases superior mesenteric artery blood flow[105], which may account for the lack of a causal role for PDAs or early indomethacin treatment to lead to early NEC[106]. A recent retrospective cohort study from a single NICU evaluated 415 infants over a 5-year period who were treated with indomethacin for PDA closure. The infants were divided into three groups based on enteral feed volume during treatment: those whose feeds were held (n=229), those whose feeds were ≤60 mL/kg/day (n=142), and those whose feeds were >60 mL/kg/day (n=44). This study found no significant difference in incidence of NEC (≥ Bell stage IIa) and noted that a preemptive reduction in enteral feeds was associated with a significantly longer time to reach full feeds[107].
As with intravenous nutrition, enteral nutrient regimens also must provide sufficient protein to meet the growth rates of the normally growing fetus of the same gestational age. Several studies have noted declining growth and head circumference Z-score changes (birth to hospital discharge) by year of study as human milk (mother’s and donor) feeding increased[108]. For infants <28-30 weeks gestation, to provide about 4.0 g/kg/day of protein, the amount needed to duplicate fetal growth, a protein concentration of ≥2.8 g/100 mL of milk must be reached. While mother’s milk primarily, but also donor milk (secondarily), are the recommended enteral feeding products for newborn infants, their nutrient concentrations generally are too low, particularly of protein, to meet the growth requirements of preterm infants. This cannot be reached with a powder fortifier; thus, protein intakes remain below adequate intake levels with use of powder fortifiers. Liquid fortifiers add greater amounts of protein (around 1.7 g of protein per 100 mL of milk) and satisfactory protein intakes are achieved most of the time. The customary formulation to do this involves adding 1 mL of liquid protein fortifier per 25 mL of milk, which will increase protein intake by 1 g/kg/day at 150 mL/kg/day of enteral feeds[109]. This approach increases protein gain and growth, as nearly all milk but particularly donor milk contains insufficient amounts of protein, especially as lactation matures[110-111]. While a systematic review did not find significant increases in the rate of NEC infants whose mother’s milk was supplemented with cow milk derived protein fortifiers[112], recent studies have documented lower rates of NEC when human milk derived protein fortifiers were used[113].
Summary and recommendationGrowth outcomes of ELBW infants remain suboptimal, because they are not fed enough, especially of protein. Early protein losses are minimized by providing 3-4 g/kg/day of amino acids; less amino acids and protein could lead to neurological deficits. In general, preterm infants have been fed excessive energy, which only makes them fatter; but they still lack essential fatty acids, particularly the LC PUFAs, particularly DHA. Providing at least ~70 (intravenous) to 90 (enteral) non-protein kcal/kg/day and 3-4 g/kg/day of amino acids or protein may approximate fetal protein accretion and growth in reasonably healthy ELBW infants. Late preterm or moderately preterm infants also receive suboptimal nutrition and do not grow optimally for the same reasons—insufficient protein and suboptimal amounts of mother’s milk with insufficient fortification. Research also is needed to determine optimal amino acid and energy supplies in sick infants and those who have experienced significant intrauterine and postnatal growth restriction.
Only a few pre-discharge intervention studies to promote growth in preterm infants during their NICU stay have information on later neurocognitive, adiposity, and insulin resistance/cardiovascular risk factor outcomes. Abundant and consistent evidence from observational studies link faster postnatal growth to better neurocognitive outcomes in preterm infants, but these studies show no obvious windows of association and there is a high risk of confounding by other factors/disease processes that affect both growth and cognition. Where associations are reported in observational studies linking faster postnatal growth to adverse cardiovascular risk markers in preterm infants, the findings have often not been adjusted for body size at the time of the outcome measurements. Moreover, comparisons were often not made to the normal ranges of these outcome parameters in unselected populations. Present evidence suggests that even brief periods of relative undernutrition during a sensitive period of development have significant adverse effects on later development[114].
It also is worth noting that there still is much yet to learn about how to optimally feed preterm infants. The balance of studies, for example, have shown that growth between birth and expected term and 12-18 months post-term has little or no significant effect on later blood pressure and metabolic syndrome, whereas reduced growth during the NICU hospitalization very clearly adversely impacts later neurodevelopment. There is, however, a paucity of well-designed, controlled studies in preterm infants of the effects of nutrition during NICU hospitalization and after discharge on neurodevelopment or the risk of developing later life disorders such as hypertension, obesity, or insulin resistance[115]. Further research is needed to determine the optimal nutrition and rate of growth in preterm infants that will achieve optimal neurocognitive benefits while minimizing the longer-term risk of chronic diseases.
Practical recommendationsNormal fetal nutrition is a reasonable guide to the nutrient requirements for preterm infants of the same gestational age. Several aspects of fetal nutrition are important to recognize. First, amino acids are pumped into the fetus by active transport in the placenta at rates that are higher than the fetus can use for protein synthesis and net protein accretion. The excess amino acid load is oxidized for energy. Secondly, glucose is taken up and used to meet energy needs according to the metabolic rate of the fetus, which does not increase significantly with excess glucose. Excess glucose not only causes hypoxia and acidosis, but also leads to various forms of toxicity that impair insulin secretion and induce cellular and systemic inflammation. Fetal nutrition, therefore, is designed for growth and to maintain energy balance. In contrast “customary” ELBW/VLBW Nutrition is aimed at an excess of energy and insufficient amounts of protein. Glucose is pumped into the infant at rates that are higher than the infant can use. The excess glucose load produces hyperglycemia, which leads to inflammation and cellular toxicity. Furthermore, amino acids are provided at rates that are less than needed for normal growth rates.
To more appropriately support growth of the preterm infant to meet the growth rates and body compositions of the normal fetus of the same gestational age, preterm neonatal nutrition should be aimed at supporting growth and maintaining energy. IV nutrition should begin right after birth, and amino acids should be infused at rates just higher than the infant needs (3-4 g/kg/day). The excess amino acid load will be oxidized, providing useful energy and diminishing hyperglycemia. Glucose should be infused to meet glucose needs, adjusting the infusion rate to maintain normal glucose concentrations (50-100 mg/dL). Maximum intravenous glucose infusion rates should not exceed 6-10 mg/kg/min = 27-42 kcal/kg/day. Lipids should be provided to meet additional energy (and EFA) needs: 2-3 g/kg/day = 18-36 kcal/kg/day. Intravenous feeding should be started right after birth.
Enteral feeding should be started as soon as the infant is stable[116-117], but for most preterm infants, mother’s colostrum should be provided within the first hour or two after birth, and buccal swabbing with colostrum should be done immediately to help establish an appropriate oral and gastrointestinal microflora in the infant[118]. Subsequent feeding should preferably use fresh mother’s milk, with donor milk an acceptable alternative. Protein (and minerals) should be used to supplement mother’s milk and especially donor milk to meet the gestational age-specific protein requirements necessary for normal fetal growth rates. Liquid fortifiers provide more protein than powder fortifiers and they do not lead to infections since they can be sterilized in contrast to the powder fortifiers.
Trophic or gut priming enteral feeding with milk should be advanced progressively according to standard protocols to avoid too many delays or stopping feedings for events, such as gastric residuals, that have no bearing on adverse clinical conditions, particularly necrotizing enterocolitis[119]. An every 3-hour feeding schedule works for most preterm infants, though there might be an advantage to every 2 hour feeds in the smallest infants. Many studies and meta-analyses have documented that there is no increase in the rate of NEC or other major morbidities when feedings were advanced at 30-35 mL/kg/day vs. slower rates of advancement(15-20 mL/kg/day[120-121]). Achieving full enteral feedings sooner reduces the risk from TPN and infections associated with invasive catheters[122].
There also is little evidence to support delayed enteral nutrition, both in terms of starting time after birth and rate of advancement, in infants who had IUGR with abnormal antenatal Doppler values, particularly absent or reversed end diastolic flow (AREDF) in the umbilical artery, in whom elective preterm delivery is common[123]. Such infants do have increased rates of NEC and feeding intolerance, which indicates that in addition to using fresh mother’s milk, feedings should not be started until the infant is physiologically stable, and should be advanced cautiously, though there is no reason to restrict early oral colostrum administration both to the buccal mucosa and into the stomach[124] .
Table 2 below is one example of a feeding advancement protocol that adjusts for gestational age and prior growth restriction.
1 概述早产儿营养补给的目标是达到同胎龄健康胎儿的正常生长速率。基本营养素的供给不足不仅会造成生长受限,同时会导致免疫防御不足而增加早产儿对感染性疾病的易感性,增加呼吸窘迫的发生率,降低损伤组织的修复能力以及影响大脑发育。神经元发育障碍可能是早产儿营养缺失所造成的最严重的后遗症,可导致认知缺陷,神经发育结局的改善与早产儿营养状况的提高相关。
根据总的能量及蛋白质需求以及个体成分如氨基酸、碳水化合物及脂肪,甚至细化到氧气的需求来优化早产儿的营养是非常必要的。临床上给早产儿提供充足的营养一直面临着挑战,特别是早产儿刚出生时依赖静脉营养的情况下,此时蛋白质储存的降低每天可达1.5%。在出生后最初5 d保证每天至少3 g/kg氨基酸摄入量可以减少极低出生体重早产儿的蛋白质分解和缺失,并可提高蛋白质平衡和生长结局。营养物质的数量与质量对于早产儿的正常生长发育(包括神经系统发育的结局)至关重要,但临床上早产儿生长发育受限的现象较普遍。早产儿在NICU住院期间的生长速率在早产儿神经系统发育结局和体型发育方面发挥了关键的、不可被取代的作用。Lucas等的研究显示,给予4周充足剂量的蛋白质及能量的早产儿,在纠正胎龄18个月时生长发育及神经发育得到明显改善,7.5岁时的认知能力高于对照组,16岁时头颅MRI影像学检查显示其头围和尾状核体积高于对照组且智商(IQ)评分较高。
然而目前的营养方案仍很难实现早产儿最佳营养管理从而达到最佳生长发育状态,因此,有必要重新评估早产儿应该达到的生长率以及实现该生长率的基本营养需求。
2 关于早产儿生长速度直接测量正常的胎儿生长是不可能的。目前根据貌似“正常”的存活早产儿间接估算的数据,妊娠24~38周胎儿体重平均增加17 g/kg/d(图 1),但实际上大多数早产儿出生后达不到宫内生长的速度(图 2),主要的原因就是营养不足(图 3)。首先,大多数早产儿出生后第一日(甚至数日)的静脉氨基酸量很低甚至没有;其次,开始肠内喂养的时间滞后;第三,大多数婴儿只能耐受有限的营养支持,如静脉氨基酸的输注率<3 g/kg/d;第四,在开始营养支持后,静脉氨基酸、脂类和肠内营养的增加都很缓慢;第五,大部分婴儿应用的是未强化母乳或必需氨基酸含量不足的完全胃肠外营养。多数情况下人们只是假设生理上尚不稳定的早产儿不能承受正常量肠外或肠内营养。
3 关于全静脉注射氨基酸:应在生后2~3 h内开始注射至少2 g/kg/d的氨基酸,<30周孕龄的早产儿应在第一个24 ~ 48 h增加至3.5~4 g/kg/d(极低出生体重儿<27周妊娠增加至4 g/kg/d)。
葡萄糖:出生后葡萄糖(10%葡萄糖液)的输注速度应以新生儿能耐受且不引起高血糖的最大速率予以输注,以防低血糖的发生。高血糖的定义为出生后任意血糖浓度>120 mg/dL。出生后1 h到几天,各种应激反应导致体内去甲肾上腺素和皮质醇的水平较高,因此葡萄糖输注过快时(> 6~8 mg/kg/min)会造成高血糖。早产儿高血糖是加重全身炎症反应的潜在因素,且高血糖的早产儿患视网膜病变的风险增加。
脂质:静脉脂质输注应在出生后24 h内开始,并提高到可耐受的2.0至3.0~3.5 g/kg/d,通常持续2~3 d。静脉中的脂质为氧化代谢提供了碳,并且提供了有利于全身细胞特别是脑组织细胞膜的合成所需的脂肪酸。但是,过多的脂质输注会导致高甘油三酯血症(定义为任意血甘油三酯浓度大于150 mg/dL),会导致脂质的不良沉积及肝细胞发生炎症,这可能也是长期静脉营养的患儿常发生胆管性黄疸和肝衰竭的原因。极低出生体重儿清除血浆脂质的能力降低,建议出生后头几天的脂质输注率为0.5~1.0 g/kg/d,特别是合并感染、严重肺疾病、外科应激、使用激素时。
热卡:第1天为50 kcal/kg/d,至第3天应达到80 kcal/kg/d。
4 关于静脉营养向肠内营养的过渡在肠外营养向肠内营养过渡期间,由于营养摄入量的暂时性下降,蛋白质和能量的摄入可能会下降。解决这一问题的办法是尽快增加肠内营养和应用母乳强化剂。母乳强化剂可在肠内喂养量达到50 mL/kg/d时就开始。一项对100个极低出生体重儿的随机研究显示,在肠内喂养量仅20 mL/kg/d的时候就开始早期添加母乳强化剂,与肠内喂养达到100 mL/kg/d后才开始添加母乳强化剂相比,早期开始添加母乳强化剂的婴儿在第1、2、3周龄的每日蛋白摄入量更高,在第4周龄的累计蛋白摄入量也更高,但两组在达到完全喂养量所需的天数、喂养耐受和坏死性小肠结肠炎发生率方面没有差异。
5 关于肠内营养长时间肠外营养可导致感染率增加(由于免疫抑制、肠道菌群失调以及中心静脉置管相关的细菌感染几率增加)、肠黏膜萎缩、肠道分泌的消化酶减少、心肝脂肪浸润增加、全身炎症反应综合征、IgA减少等。在成人和年长儿、足月儿的研究中,当肠内营养受限而静脉营养过多时,多种疾病的发生率或病死率均增加。这些问题很可能是过多输注葡萄糖导致高血糖的结果。尽早开始肠内营养,同时适度给予静脉营养才是有益的。
与静脉营养一样,肠内营养应提供可以满足相同胎龄胎儿正常生长发育所需的蛋白质。对于胎龄<28~30周的婴儿,必须提供≥2.9 g/100 mL蛋白浓度的奶,以满足胎儿生长所需的4.0 g/kg/d的蛋白质。尽管母亲的母乳(首选)或供者的母乳(次选)都是新生儿肠内营养所推荐的食物,但它们的营养素浓度特别是蛋白质的浓度太低,不能满足早产儿的生长需求。液体母乳强化剂与粉状母乳强化剂相比,液体强化剂可以添加的蛋白质较多(每100 mL牛奶大约1.7 g蛋白质),通常可达到满意的蛋白摄入量。
液态母乳强化剂的添加量为每25 mL奶加1 mL母乳强化剂,肠内喂养150 mL/kg/d添加了强化剂的母乳,可提高1 g/kg/d的蛋白摄入量。系统回顾研究并未发现牛奶来源的母乳强化剂可导致婴儿坏死性小肠结肠炎的发生率提高。最新研究发现,使用人乳来源的母乳强化剂时婴儿坏死性小肠结肠炎的发生率更低。
6 其他建议胎儿营养是专为生长和维持能量平衡设计的,应注意到胎儿营养的以下几个方面的问题:首先,氨基酸是在胎盘以主动转运的方式摄入到胎儿体内的,其速率高于胎儿蛋白质合成及净蛋白累积所需;其次,葡萄糖的摄入和利用依据的是胎儿代谢所需的热卡。为了更好地维持早产儿的生长以达到相同胎龄儿的生长速率和身体成分,早产儿的营养应旨在支持生长和维持能量。静脉营养应在出生后立即开始,并且氨基酸的输注率应稍高于婴儿所需(3~4 g/kg/d),这样有助于减少高血糖的发生。葡萄糖的供给应满足机体需要、维持正常的血糖浓度(50~100 mg/dL),葡萄糖静脉输注的最大速度应不超过10 mg/kg/min(27~42 kcal/kg/d)。脂质供给应满足额外的能量的需要:2~3 g/kg/d(18~36 kcal/kg/d)。
肠内喂养应在婴儿稳定后尽早开始。对于大多数早产儿,在出生后的1~2 h内可予以口服初乳,并且立即再在口颊部位用初乳进行涂抹,这样做将有助于早产儿建立正常的口腔和肠道菌群。后续的喂养则最好使用新鲜的母乳,在亲母母乳不足时,也可选用捐赠母乳。可在母乳中添加母乳强化剂以增加蛋白质和矿物质的含量,液体母乳强化剂比粉末母乳强化剂提供的蛋白质更多且不会导致感染,因为液体母乳强化剂可被消毒而粉末状母乳强化剂不易消毒。
肠内喂养量应按照喂养指南的要求逐步增加,要尽量避免因婴儿存在胃残留, 或担心坏死性小肠结肠炎的发生而延迟或停止肠内营养。每3 h喂一次的方法适用于大部分早产儿。关于加奶速度,临床研究和Meta分析结果显示,以30~35 mL/kg/d的速度增加肠内喂养量与以15~20 mL/kg/d的速度增加肠内喂养量相比,坏死性小肠结肠炎的发生率并没有增加。对于产前脐血彩超不正常(特别是脐动脉血液断流或者母胎输血)的宫内生长受限患儿,尽管坏死性小肠结肠炎或喂养不耐受的风险会增加,但目前没有证据显示对这部分早产儿需延迟开奶或减慢肠内喂养添加速度。所有早产儿都可以尽早开始应用初乳进行口腔颊黏膜的擦拭。
| [1] | American Academy of Pediatrics. Committee on Nutrition:Nutritional needs of low-birth-weight infants[J]. Pediatrics, 1985, 75 (5): 976–985. |
| [2] | Hobar JD, Ehrenkranz RA, Badger GJ, et al. Weight growth velocity and postnatal growth failure in infants 501 to 1500 grams:2000-2013[J]. Pediatrics, 2015, 136 (1): e84–e92. DOI:10.1542/peds.2015-0129 |
| [3] | Lafeber HN, Westerbeek EA, van den Berg A, et al. Nutritional factors influencing infections in preterm infants[J]. J Nutr, 2008, 138 (9): 1813S–1817S. |
| [4] | Bhatia J, Parish A. Nutrition and the lung[J]. Neonatology, 2009, 95 (4): 362–367. DOI:10.1159/000209302 |
| [5] | Commare CE, Tappenden KA. Development of the infant intestine:implications for nutrition support[J]. Nutr Clin Pract, 2007, 22 (2): 159–173. DOI:10.1177/0115426507022002159 |
| [6] | Berni Canani R, Passariello A, Buccigrossi V, et al. The nutritional modulation of the evolving intestine[J]. J Clin Gastroenterol, 2008, 42 (Suppl 3 Pt 2): S197–S200. |
| [7] | Yau KI, Chang MH. Growth and body composition of preterm, small-for-gestational-age infants at a postmenstrual age of 37-40 weeks[J]. Early Hum Dev, 1993, 33 (2): 117–131. DOI:10.1016/0378-3782(93)90207-B |
| [8] | Smart JL. Vulnerability of developing brain to undernutrition[J]. Ups J Med Sci, 1990, 48 (Suppl): 21–41. |
| [9] | Smart JL. Critical periods in brain development[M]//Bock GR, Whelan J. The Childhood Environment and Adult Disease. Ciba Found Symp, Chichester. Wiley, 1991:109-128. Smart JL. Critical periods in brain development[M]//Bock GR, Whelan J. The Childhood Environment and Adult Disease. Ciba Found Symp, Chichester. Wiley, 1991:109-128. |
| [10] | Georgieff MK, Hoffman JS, Pereira GR, et al. Effect of neonatal caloric deprivation on head growth and 1-year developmental status in preterm infants[J]. J Pediatr, 1985, 107 (4): 581–587. DOI:10.1016/S0022-3476(85)80028-7 |
| [11] | Hack M, Breslau N, Weissman B, et al. Effect of very low birth weight and subnormal head size on cognitive abilities at school age[J]. N Engl J Med, 1991, 325 (4): 231–237. DOI:10.1056/NEJM199107253250403 |
| [12] | Ghods E, Kreissl A, Brandstetter S, et al. Head circumference catch-up growth among preterm very low birth weight infants:effect on neurodevelopmental outcome[J]. J Perinat Med, 2011, 39 (5): 579–586. |
| [13] | Ehrenkranz RA, Dusick AM, Vohr BR, et al. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants[J]. Pediatrics, 2006, 117 (4): 1253–1261. DOI:10.1542/peds.2005-1368 |
| [14] | Belfort MB, Kuban KC, O'Shea TM, et al. Weight status in the first 2 years of life and neurodevelopmental impairment in extremely low gestational age newborns[J]. J Pediatr, 2016, 168 (e2): 30–35. |
| [15] | Lodygensky GA, Seghier ML, Warfield SK, et al. Intrauterine growth restriction affects the preterm infant's hippocampus[J]. Pediatr Res, 2008, 63 (4): 438–443. DOI:10.1203/PDR.0b013e318165c005 |
| [16] | Isaacs EB, Gadian DG, Sabatini S, et al. The effect of early human diet on caudate volumes and IQ[J]. Pediatr Res, 2008, 63 (4): 229–231. |
| [17] | Belfort MB, Rifas-Shiman SL, Sullivan T, et al. Infant growth before and after term:effects on neurodevelopment in preterm infants[J]. Pediatrics, 2011, 128 (4): e899–e906. DOI:10.1542/peds.2011-0282 |
| [18] | Vasu V, Modi N. Assessing the impact of preterm nutrition[J]. Early Hum Dev, 2007, 83 (12): 813–818. DOI:10.1016/j.earlhumdev.2007.09.008 |
| [19] | Denne SC. Early nutritional support for extremely premature infants:what amino acid amount should be given?[J]. Am J Clin Nutr, 2016, 103 (6): 1383–1384. DOI:10.3945/ajcn.116.136119 |
| [20] | Denne SC, Poindexter BB. Evidence supporting early nutritional support with parenteral amino acid infusion[J]. Semin Perinatol, 2007, 31 (2): 56–60. DOI:10.1053/j.semperi.2007.02.005 |
| [21] | Van Goudoever JB, Colen T, Wattimena JL, et al. Immediate commencement of amino acid supplementation in preterm infants:effect on serum amino acid concentrations and protein kinetics on the first day of life[J]. J Pediatr, 1995, 127 (3): 458–465. DOI:10.1016/S0022-3476(95)70083-8 |
| [22] | Thureen PJ, Melara D, Fennessey PV, et al. Effect of low versus high intravenous amino acid intake on very low birth weight infants in the early neonatal period[J]. Pediatr Res, 2003, 53 (1): 24–32. DOI:10.1203/00006450-200301000-00008 |
| [23] | Poindexter BB, Langer JC, Dusick AM, et al. Early provision of parenteral amino acids in extremely low birth weight infants:relation to growth and neurodevelopmental outcome[J]. J Pediatr, 2006, 148 (3): 300–305. DOI:10.1016/j.jpeds.2005.10.038 |
| [24] | Cormack BE, Bloomfield FH. Increased protein intake decreases postnatal growth faltering in ELBW babies[J]. Arch Dis Child Fetal Neonatal Ed, 2013, 98 (5): F399–F404. DOI:10.1136/archdischild-2012-302868 |
| [25] | Bolisetty S, Pharande P, Nirthanakumaran L, et al. Improved nutrient intake following implementation of the consensus standardised parenteral nutrition formulations in preterm neonates:a before-after intervention study[J]. BMC Pediatr, 2014, 14 : 309–315. DOI:10.1186/s12887-014-0309-0 |
| [26] | Wilson DC, Cairns P, Halliday HL, et al. Randomised controlled trial of an aggressive nutritional regimen in sick very low birthweight infants[J]. Arch Dis Child Fetal Neonatal Ed, 1997, 77 (1): F4–F11. DOI:10.1136/fn.77.1.F4 |
| [27] | Morgan C, McGowan P, Herwitker S, et al. Postnatal head growth in preterm infants:a randomized controlled parenteral nutrition study[J]. Pediatrics, 2014, 133 (1): e120–e128. DOI:10.1542/peds.2013-2207 |
| [28] | Hay WW Jr, Ziegler EE. Growth failure among preterm infants is not innocuous and must be prevented. Commentary[J]. J Perinatol, 2016, 36 (7): 500–502. DOI:10.1038/jp.2016.85 |
| [29] | Poindexter B. Approaches to growth faltering[M]//Koletzko B, Poindexter B, Uauy R. Nutritional Care of Preterm Infants. Karger:Basel, 2014:228-238. Poindexter B. Approaches to growth faltering[M]//Koletzko B, Poindexter B, Uauy R. Nutritional Care of Preterm Infants. Karger:Basel, 2014:228-238. |
| [30] | Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network[J]. Pediatrics, 2010, 126 (3): 443–456. DOI:10.1542/peds.2009-2959 |
| [31] | Morgane PJ, Mokler DJ, Galler JR. Effects of prenatal protein malnutrition on the hippocampal formation[J]. Neurosci Biobehav Rev, 2002, 26 (4): 471–483. DOI:10.1016/S0149-7634(02)00012-X |
| [32] | Lucas A, Morley R, Cole TJ, et al. Early diet in preterm babies and developmental status at 18 months[J]. Lancet, 1990, 335 (8704): 1477–1481. DOI:10.1016/0140-6736(90)93026-L |
| [33] | Lucas A, Morley R, Cole TJ. Randomised trial of early diet in preterm babies and later intelligence quotient[J]. BMJ, 1998, 317 (7171): 1481–1487. DOI:10.1136/bmj.317.7171.1481 |
| [34] | Isaacs EB, Morley R, Lucas A. Early diet and general cognitive outcome at adolescence in children born at or below 30 weeks gestation[J]. J Pediatr, 2009, 155 (2): 229–234. DOI:10.1016/j.jpeds.2009.02.030 |
| [35] | Tan MJ, Cooke RW. Improving head growth in very preterm infants-a randomised controlled trial I:neonatal outcomes[J]. Arch Dis Child Fetal Neonatal Ed, 2008, 93 (5): F337–F341. DOI:10.1136/adc.2007.124230 |
| [36] | Tan M, Abernethy L, Cooke R. Improving head growth in preterm infants-a randomised controlled trial Ⅱ:MRI and developmental outcomes in the first year[J]. Arch Dis Child Fetal Neonatal Ed, 2008, 93 (5): F342–F346. DOI:10.1136/adc.2007.124255 |
| [37] | Stephens BE, Walden RV, Gargus RA, et al. First-week protein and energy intakes are associated with 18-month developmental outcomes in extremely low birth weight infants[J]. Pediatrics, 2009, 123 (5): 1337–1343. DOI:10.1542/peds.2008-0211 |
| [38] | Naeye R, Dixon J. Distortions in fetal growth standards[J]. Pediatr Res, 1978, 12 (10): 987–991. DOI:10.1203/00006450-197810000-00008 |
| [39] | Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants[J]. BMC Pediatr, 2003, 13 : 59. |
| [40] | Olsen IE, Groveman SA, Lawson ML, et al. New intrauterine growth curves based on United States data[J]. Pediatrics, 2010, 125 (2): e214–e224. DOI:10.1542/peds.2009-0913 |
| [41] | Carlson SJ, Ziegler EE. Nutrient intakes and growth of very low birth weight infants[J]. J Perinatol, 1998, 18 (4): 252–258. |
| [42] | Ehrenkranz RA, Younes N, Lemons JA, et al. Longitudinal growth of hospitalized very low birth weight infants[J]. Pediatrics, 1999, 104 (2 Pt 1): 280–289. |
| [43] | Dancis J, O'connell Jr, Holt LE Jr. A grid for recording the weight of premature infants[J]. J Pediatrics, 1948, 33 (1): 570–572. |
| [44] | Dinerstein A, Solana CL, Perez GP, et al. Early and aggressive nutritional strategy (parenteral and enteral) decreases postnatal growth failure in very low birth weight infants[J]. J Perinatol, 2006, 26 (7): 436–442. DOI:10.1038/sj.jp.7211539 |
| [45] | Kitanaka T, Alonso JG, Gilbert RD, et al. Fetal responses to long-term hypoxemia in sheep[J]. Am J Physiol, 1989, 256 (6 Pt 2): R1348–R1354. |
| [46] | Stockman JA, Clark DA. Weight gain:a response to transfusion in selected preterm infants[J]. Am J Dis Child, 1984, 138 (9): 828–830. |
| [47] | Carver TD, Anderson SM, Aldoretta PA, et al. Glucose suppression of insulin secretion in chronically hyperglycemic fetal sheep[J]. Pediatr Res, 1995, 38 (5): 754–762. DOI:10.1203/00006450-199511000-00020 |
| [48] | Hay WW Jr, Sparks JW, Quissell BJ, et al. Simultaneous measurements of umbilical uptake, fetal utilization rate, and fetal turnover rate of glucose[J]. Am J Physiol, 1981, 240 (6): E662–E668. |
| [49] | Zarlengo KM, Battaglia FC, Fennessey P, et al. Relationship between glucose utilization rate and glucose concentration in preterm infants[J]. Biol Neonate, 1986, 49 (4): 181–189. DOI:10.1159/000242529 |
| [50] | Sunehag A, Ewald U, Larsson A, et al. Glucose production rate in extremely immature neonates (<28 weeks) studied by use of deuterated glucose[J]. Pediatr Res, 1993, 33 (2): 97–100. DOI:10.1203/00006450-199302000-00001 |
| [51] | Marconi AM, Paolini C, Buscaglia M, et al. The impact of gestational age and fetal growth on the maternal-fetal glucose concentration difference[J]. Obstet Gynecol, 1996, 87 (6): 937–942. DOI:10.1016/0029-7844(96)00048-8 |
| [52] | Srinivasan G, Pildes RS, Cattamanchi G, et al. Plasma glucose values in normal neonates:a new look[J]. J Pediatr, 1986, 109 (1): 114–117. DOI:10.1016/S0022-3476(86)80588-1 |
| [53] | Helms RA, Mauer EC, Hay WW Jr, et al. Effect of intravenous L-carnitine on growth and fat metabolism parameters during parenteral nutrition in neonates[J]. JPEN J Parenter Enteral Nutr, 1990, 14 (5): 448–453. DOI:10.1177/0148607190014005448 |
| [54] | Sellmayer A, Koletzko B. Long-chain polyunsaturated fatty acids and eicosanoids in infants-Physiological and pathophysiological aspects and open questions[J]. Lipids, 1999, 34 (2): 199–205. DOI:10.1007/s11745-999-0354-z |
| [55] | Carlson SE. Docosahexaenoic acid and arachidonic acid in infant development[J]. Semin Neonatol, 2001, 6 (5): 437–449. DOI:10.1053/siny.2001.0093 |
| [56] | Chacko SK, Ordonez J, Sauer PJ, et al. Gluconeogenesis is not regulated by either glucose or insulin in extremely low birth weight infants receiving total parenteral nutrition[J]. J Pediatr, 2011, 158 (6): 891–896. DOI:10.1016/j.jpeds.2010.12.040 |
| [57] | Lapillonne A, Eleni dit Trolli SE, Kermorvant-Duchemin E. Postnatal DHA deficiency is an inevitable consequence of current recommendations and practices in preterm infants[J]. Neonatology, 2010, 98 (4): 397–403. DOI:10.1159/000320159 |
| [58] | Tam EWY, Chau V, Barkovich AJ, et al. Early postnatal docosahexaenoic acid levels and improved preterm brain development[J]. Pediatr Res, 2016, 79 (5): 723–730. DOI:10.1038/pr.2016.11 |
| [59] | Carlson SE, Werkman SH, Rhodes PG, et al. Visual acuity development in healthy preterm infants:effect of marine oil supplementation[J]. Am J Clin Nutr, 1993, 58 (1): 35–42. |
| [60] | Micheli JL, Schutz Y. Protein[M]//Tsang RC. Nutritional Needs of the Preterm Infant. Pawling, NY:Caduceus Medical Publishers, 1993:29-46. Micheli JL, Schutz Y. Protein[M]//Tsang RC. Nutritional Needs of the Preterm Infant. Pawling, NY:Caduceus Medical Publishers, 1993:29-46. |
| [61] | Kashyap S, Forsyth M, Zucker C, et al. Effects of varying protein and energy intakes on growth and metabolic response in low birth weight infants[J]. J Pediatr, 1986, 108 (6): 955–963. DOI:10.1016/S0022-3476(86)80940-4 |
| [62] | Vasu V, Thomas EL, Durighel G, et al. Early nutritional determinants of intrahepatocellular lipid deposition in preterm infants at term age[J]. Int J Obes (Lond), 2013, 37 (4): 500–504. DOI:10.1038/ijo.2012.213 |
| [63] | Olsen IE, Lawson ML, Ferguson AN, et al. BMI curves for preterm infants[J]. Pediatrics, 2015, 135 (3): e572–e581. DOI:10.1542/peds.2014-2777 |
| [64] | Ziegler EE, O'Donnell AM, Nelson SE, et al. Body composition of the reference fetus[J]. Growth, 1976, 40 (4): 329–341. |
| [65] | Embleton ND. Optimal protein and energy intakes in preterm infants[J]. Early Human Dev, 2007, 83 (12): 831–837. DOI:10.1016/j.earlhumdev.2007.10.001 |
| [66] | Thureen PJ, Anderson AH, Baron KA, et al. Protein balance in the first week of life in ventilated neonates receiving parenteral nutrition[J]. Am J Clin Nutr, 1998, 68 (5): 1128–1135. |
| [67] | Morgan C. Early amino acid administration in very preterm infants:Too little, too late, or too much, too soon?[J]. Semin Fetal Neonatal Med, 2013, 18 : 160–165. DOI:10.1016/j.siny.2013.02.002 |
| [68] | Uthaya S, Modi N. Practical preterm parenteral nutrition:systematic literature review and recommendations for practice[J]. Early Human Dev, 2014, 90 (11): 747–753. DOI:10.1016/j.earlhumdev.2014.09.002 |
| [69] | Clark RH, Chace DH, Spitzer AR; Pediatrix Amino Acid Study Group. Effects of two different doses of amino acid supplementation on growth and blood amino acid levels in premature neonates admitted to the neonatal intensive care unit:a randomized, controlled trial[J]. Pediatrics, 2007, 120 (6): 1286–1296. DOI:10.1542/peds.2007-0545 |
| [70] | Thureen PJ, Hay WW. Early aggressive nutrition in preterm infants[J]. Semin Neonatol, 2001, 6 (5): 403–415. DOI:10.1053/siny.2001.0061 |
| [71] | Embleton ND, Morgan C, King C. Balancing the risks and benefits of parenteral nutrition for preterm infants:can we define the optimal composition[J]. Arch Dis Child Fetal Neonatal Ed, 2015, 100 (1): F72–F75. DOI:10.1136/archdischild-2013-304061 |
| [72] | Brown LD, Rozance PJ, Thorn SR, et al. Acute supplementation of amino acids increases net protein accretion in IUGR fetal sheep[J]. Am J Physiol Endocrinol Metab, 2012, 303 (3): E352–E364. DOI:10.1152/ajpendo.00059.2012 |
| [73] | Sunehag AL. Hyperglycemia is a risk factor for early death and morbidity in extremely low birth-weight infants[J]. Pediatrics, 2006, 118 (5): 1811–1818. DOI:10.1542/peds.2006-0628 |
| [74] | Stensvold HJ, Strommen K, Lang AM, et al. Early enhanced parenteral nutrition, hyperglycemia, and death among extremely low-birth-weight infants[J]. JAMA Pediatr, 2015, 169 (11): 1003–1010. DOI:10.1001/jamapediatrics.2015.1667 |
| [75] | Picard M, Juster RP, McEwen BS. Mitochondrial allostatic load puts the ‘gluc’ back in glucocorticoids[J]. Nat Rev Endocrinol, 2014, 10 (5): 303–310. DOI:10.1038/nrendo.2014.22 |
| [76] | Stoll B, Horst DA, Cui L, et al. Chronic parenteral nutrition induces hepatic inflammation, steatosis, and insulin resistance in neonatal pigs[J]. J Nutr, 2010, 140 (12): 2193–2200. DOI:10.3945/jn.110.125799 |
| [77] | Chavez-Valdez R, McGowan J, Cannon E, et al. Contribution of early glycemic status in the development of severe retinopathy of prematurity in a cohort of ELBW infants[J]. J Perinatology, 2011, 31 (12): 749–756. DOI:10.1038/jp.2011.19 |
| [78] | Mohsen L, Abou-Alam M, El-Dib M, et al. A prospective study on hyperglycemia and retinopathy of prematurity[J]. J Perinatology, 2014, 34 (6): 453–457. DOI:10.1038/jp.2014.49 |
| [79] | Garg R, Agthe AG, Donohue PK, et al. Hyperglycemia and Retinopathy of Prematurity in Very Low Birth Weight Infants[J]. J Perinatology, 2003, 23 (3): 186–194. DOI:10.1038/sj.jp.7210879 |
| [80] | Basu SK, Kaiser JR, Guffey D, et al. Hypoglycaemia and hyperglycaemia are associated with unfavourable outcome in infants with hypoxic ischaemic encephalopathy:a post hoc analysis of the CoolCap Study[J]. Arch Dis Child Fetal Neonatal Ed, 2016, 101 (2): F149–F155. DOI:10.1136/archdischild-2015-308733 |
| [81] | Scheurer JM, Gray HL, Demerath EW, et al. Diminished growth and lower adiposity in hyperglycemic very low birth weight neonates at 4 months corrected age[J]. J Perinatol, 2016, 36 (2): 145–150. DOI:10.1038/jp.2015.154 |
| [82] | Hay WW Jr, Brown LD, Rozance PJ, et al. Challenges in nourishing the intrauterine growth-restricted foetus-Lessons learned from studies in the intrauterine growth-restricted foetal sheep[J]. Acta Paediatr, 2016, 105 (8): 881–889. DOI:10.1111/apa.2016.105.issue-8 |
| [83] | Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, et al. A randomised controlled trial of early insulin therapy in very low birth weight infants, "NIRTURE" (neonatal insulin replacement therapy in Europe)[J]. BMC Pediatr, 2007, 7 : 29. DOI:10.1186/1471-2431-7-29 |
| [84] | Sadhwani A, Asaro LA, Goldberg C, et al. Impact of tight glycemic control on neurodevelopmental outcomes at 1 year of age for children with congenital heart disease:a randomized controlled trial[J]. J Pediatrics, 2016, 174 : 193–198. DOI:10.1016/j.jpeds.2016.03.048 |
| [85] | Bottino M, Cowett RM, Sinclair JC. Interventions for treatment of neonatal hyperglycemia in very low birth weight infants[J]. Cochrane Database Syst Rev, 2009 (1): CD007453. |
| [86] | Meetze W, Bowsher R, Compton J, et al. Hyperglycemia in extremely-low-birth-weight infants[J]. Biol Neonate, 1998, 74 (3): 214–21. DOI:10.1159/000014027 |
| [87] | Burattini I, Bellagamba MP, Spagnoli C, et al. Marche Neonatal Network. Targeting 2.5 versus 4 g/kg/day of amino acids for extremely low birth weight infants:a randomized clinical trial[J]. J Pediatr, 2013, 163 (5): 1278–1282. DOI:10.1016/j.jpeds.2013.06.075 |
| [88] | Wang H, Khaoustov VI, Krishnan B, et al. Total parenteral nutrition induces liver steatosis and apoptosis in neonatal piglets[J]. J Nutr, 2006, 136 (10): 2547–2552. |
| [89] | Clark RH, Chace DH, Spitzer AR. Effects of two different doses of amino acid supplementation on growth and blood amino acid levels in premature neonates admitted to the neonatal intensive care unit:a randomized, controlled trial[J]. Pediatrics, 2007, 120 (6): 1286–1296. DOI:10.1542/peds.2007-0545 |
| [90] | Uthaya S, Liu X, Babalis D, et al. Nutritional evaluation and optimisation in neonates:a randomized, double-blind controlled trial of amino acid regimen and intravenous lipid composition in preterm parenteral nutrition[J]. Am J Clin Nutr, 2016, 103 (6): 1443–1452. DOI:10.3945/ajcn.115.125138 |
| [91] | Morgan C, Burgess L. High protein intake does not prevent low plasma levels of conditionally essential amino acids in very preterm infants receiving parenteral nutrition[J]. JPEN J Parenter Enteral Nutr, 2015, Jul 6. pii:0148607115594009, Epub ahead of print. Morgan C, Burgess L. High protein intake does not prevent low plasma levels of conditionally essential amino acids in very preterm infants receiving parenteral nutrition[J]. JPEN J Parenter Enteral Nutr, 2015, Jul 6. pii:0148607115594009, Epub ahead of print. |
| [92] | Senterre T, Rigo J. Optimizing early nutritional support based on recent recommendations in VLBW infants and postnatal growth restriction[J]. J Pediatr Gastroenterol Nutr, 2011, 53 (5): 536–542. |
| [93] | Miller M, Vaidya R, Rastogi D, et al. From parenteral to enteral nutrition:a nutrition-based approach for evaluation of postnatal growth failure in preterm infants[J]. J Parenter Enteral Nutr, 2014, 38 (4): 489–497. DOI:10.1177/0148607113487926 |
| [94] | The SIFT Investigators Group. Early enteral feeding strategies for very preterm infants:current evidence from Cochrane reviews[J]. Arch Dis Child Fetal Neonatal Ed, 2013, 98 (6): F470–F472. DOI:10.1136/archdischild-2012-303260 |
| [95] | Shah SD, Dereddy N, Jones TL, et al. Early versus delayed human milk fortification in very low birth weight infants-a randomized controlled trial[J]. J Pediatr, 2016, 174 : 126–131. DOI:10.1016/j.jpeds.2016.03.056 |
| [96] | Casaer MP, Mesotten D, Hermans G, et al. Early versus late parenteral nutrition in critically ill adults[J]. N Engl J Med, 2011, 365 (6): 506–517. DOI:10.1056/NEJMoa1102662 |
| [97] | Gramlich L, Kichian K, Pinilla J, et al. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients? A systematic review of the literature[J]. Nutrition, 2004, 20 (10): 843–848. DOI:10.1016/j.nut.2004.06.003 |
| [98] | Seres DS, Valcarcel M, Guillaume M. Advantages of enteral nutrition over parenteral nutrition[J]. Therap Adv Gastroenterol, 2013, 6 (2): 157–167. DOI:10.1177/1756283X12467564 |
| [99] | Fivez T, Kerklaan D, Mesotten D, et al. Early versus late parenteral nutrition in critically ill children[J]. N Engl J Med, 2016, 374 (12): 1111–1122. DOI:10.1056/NEJMoa1514762 |
| [100] | Srinivasan V, Agus MS. Tight glucose control in critically ill children-a systematic review and metab-analysis[J]. Pediatr Diabetes, 2014, 15 (2): 75–83. DOI:10.1111/pedi.2014.15.issue-2 |
| [101] | Ohnishi S, Ichiba H, Tanaka Y, et al. Early and intensive nutritional strategy combining parenteral and enteral feeding promotes neurodevelopment and growth at 18months of corrected age and 3years of age in extremely low birth weight infants[J]. Early Hum Dev, 2016, 100 : 35–41. DOI:10.1016/j.earlhumdev.2016.03.014 |
| [102] | Liu J, Kong K, Tao Y, et al. Optimal timing for introducing enteral nutrition in the neonatal intensive care unit[J]. Asia Pac J Clin Nutr, 2015, 24 (2): 219–226. |
| [103] | Owens L, Burrin DG, Berseth CL. Minimal enteral feeding induces maturation of intestinal motor function but not mucosal growth in neonatal dogs[J]. J Nutr, 2002, 132 (9): 2717–2722. |
| [104] | Morgan J, Young L, McGuire W. Delayed introduction of progressive enteral feeds to prevent necrotizing enterocolitis in very low birth weight infants[J]. Cochrane Database Syst Rev, 2011, 16 (3): CD001970. |
| [105] | Niinikoski H, Stoll B, Guan X, et al. Onset of small intestinal atrophy is associated with reduced intestinal blood flow in TPN-fed neonatal piglets[J]. J Nutr, 2004, 134 (6): 1467–1474. |
| [106] | Clyman R, Wickremasinghe A, Jhaveri N, et al. Ductus Arteriosus Feed or Fast with Indomethacin or Ibuprofen (DAFFⅡ) Investigators. Enteral feeding during indomethacin and ibuprofen treatment of a patent ductus arteriosus[J]. J Pediatr, 2013, 163 (2): 406–411. DOI:10.1016/j.jpeds.2013.01.057 |
| [107] | Louis D, Torgalkar R, Shah J, et al. Enteral feeding during indomethacin treatment for patent ductus arteriosus:association with gastrointestinal outcomes[J]. J Perinatol, 2016, 36 (7): 544–548. DOI:10.1038/jp.2016.11 |
| [108] | Chowning R, Radmacher P, Lewis S, et al. A retrospective analysis of the effect of human milk on prevention of necrotizing enterocolitis and postnatal growth[J]. J Perinatol, 2016, 36 (3): 221–224. DOI:10.1038/jp.2015.179 |
| [109] | Ziegler EE. Human milk and human milk fortifiers[J]. World Rev Nutr Diet, 2014, 110 : 215–227. DOI:10.1159/000358470 |
| [110] | Arslanoglu S, Moro GE, Ziegler EE; The Wapm Working Group On Nutrition. Optimization of human milk fortification for preterm infants:new concepts and recommendations[J]. J Perinat Med, 2010, 38 (3): 233–238. |
| [111] | Rochow N, Fusch G, Choi A, et al. Target fortification of breast milk with fat, protein, and carbohydrates for preterm infants[J]. J Pediatrics, 2013, 163 (4): 1001–1007. DOI:10.1016/j.jpeds.2013.04.052 |
| [112] | Kuschel CA, Harding JE. Multicomponent fortified human milk for promoting growth in preterm infants[J]. Cochrane Database Syst Rev, 2004 (1): CD000343. |
| [113] | Sullivan S, Schanler RJ, Kim JH, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products[J]. J Pediatr, 2010, 156 (4): 562–e1-567.e1. DOI:10.1016/j.jpeds.2009.10.040 |
| [114] | Ong KK, Kennedy K, Castañeda-Gutiérrez E, et al. Postnatal growth in preterm infants and later health outcomes:a systematic review[J]. Acta Paediatr, 2015, 104 (10): 974–986. DOI:10.1111/apa.13128 |
| [115] | Lapillone A, Griffin IJ. Feeding preterm infants today for later metabolic and cardiovascular outcomes[J]. J Pediatr, 2013, 162 (3 Suppl): S7–S16. |
| [116] | Leaf A. Introducing enteral feeds in the high-risk preterm infant[J]. Semin Fetal Neonatal Med, 2013, 18 : 150–154. DOI:10.1016/j.siny.2013.03.002 |
| [117] | Dutta S, Singh B, Chessell L, et al. Guidelines for feeding very low birth weight infants[J]. Nutrients, 2015, 7 (1): 423–442. DOI:10.3390/nu7010423 |
| [118] | Rodriguez NA, Meier PP, Groer MW, et al. A pilot study to determine the safety and feasibility of oropharyngeal administration of own mother's colostrum to extremely low-birth-weight infants[J]. Adv Neonatal Care, 2010, 10 (4): 206–212. DOI:10.1097/ANC.0b013e3181e94133 |
| [119] | Rochow N, Fusch G, Muhlinghaus A, et al. A nutritional program to improve outcome of very low birth weight infants[J]. Clin Nutr, 2012, 31 (1): 124–131. DOI:10.1016/j.clnu.2011.07.004 |
| [120] | Morgan J, Young L, McGuire W. Slow advancement of enteral feed volumes to prevent necrotizing enterocolitis in very low birth weight infants[J]. Cochrane Database Syst Rev, 2011, 16 (3): CD001241. |
| [121] | Dorling J. How quickly should we aim for full milk feeds?[J]. Infant, 2012, 8 : 167168. |
| [122] | Härtel C, Haase B, Browning-Carmo K, et al. Does the enteral feeding advancement affect short-term outcomes in very low birth weight infants?[J]. J Pediatr Gastroenterol Nutr, 2009, 48 (4): 464–470. DOI:10.1097/MPG.0b013e31818c5fc3 |
| [123] | Mihatsch WA, Pohlandt F, Franz AR, et al. Early feeding advancement in very low-birth-weight infants with intrauterine growth retardation and increased umbilical artery resistance[J]. J Pediatr Gastroenterol Nutr, 2002, 35 (2): 144–148. DOI:10.1097/00005176-200208000-00008 |
| [124] | Dorling J, Kempley S, Leaf A. Feeding growth restricted preterm infants with abnormal antenatal Doppler results[J]. Arch Dis Child Fetal Neonatal Ed, 2005, 90 (5): F359–F363. DOI:10.1136/adc.2004.060350 |
 2017, Vol. 19
2017, Vol. 19