脓毒症是由感染引起的全身炎症反应综合征[1],其病情凶险,病死率极高,住院费用昂贵,发病率逐年增加,是重症监护病房危重患者死亡的主要原因[2]。早期诊断脓毒症并实施有效的干预措施可使脓毒症的病死率大大降低[3]。由于缺乏有效的诊断脓毒症的生物标志物,早期诊断脓毒症具有挑战性。目前脓毒症的诊断仍然是临床诊断为主,C反应蛋白(C reactive protein, CRP)和降钙素原(procalcitonin, PCT)[4]作为炎性指标用于脓毒症的诊断和预后判断已得到广泛认可,然而,这些生物标志物的地位正受到威胁[5-6],在许多非感染性疾病如恶性肿瘤、急性冠状动脉综合征、严重的创伤[7]、烧伤、心脏骤停[8]、心脏移植[9]等患者CRP和PCT也增高。因此,寻找脓毒症诊断和判断预后的新的生物标志物已成为目前急需解决的问题,也是近几年的研究热点[10-11]。
吗啡是最早从罂粟鸦片中提炼的已知最强的镇痛生物碱之一,是常用的镇痛药[12-13]。有研究发现健康人体中无法检测到内生吗啡,全身炎症反应综合征患者中内生吗啡轻度升高,而在脓毒症和脓毒症休克患者中呈显著升高,内生吗啡可能在脓毒症免疫反应机制中发挥重要作用[14]。本文通过脓毒症临床病例,用质谱法检测其血浆内生吗啡浓度,评估其对脓毒症患儿休克、死亡及多器官功能障碍(MODS)风险的预测价值,探讨内生吗啡能否成为儿童脓毒症的诊断和预后判断的生物标志物。
1 资料与方法 1.1 一般资料及分组选取2015年10月至2017年12月期间于中南大学湘雅三医院儿科住院的脓毒症患儿31例为研究对象,均符合脓毒症的诊断标准[1],其中男16例,女15例,年龄1个月至14岁。儿童感染相关器官衰竭评分(pSOFA)参照文献[15]。另选取同期16例普通感染患儿设为普通感染组(n=16),同期健康管理中心行体检的健康儿童31例为健康对照组(n=31)。各组年龄、性别、民族等比较差异均无统计学意义(P > 0.05)。本研究经过医院医学伦理委员会批准,研究对象随时可以根据自己的意愿退出临床观察。根据入院后1个月内是否死亡统计病死率。
1.2 检测指标所有患儿入院24 h内,健康儿童行体检时,使用一次性采血管(肝素锂抗凝管)抽取外周静脉血2 mL,立即于冷冻高速离心机(4℃,3 758 r/min)离心10 min后取上清液置于-80℃冰箱保存[16]。用高效液相质谱串联法检测各组血浆内生吗啡浓度。吗啡标准品从中国食品药品检定研究院购买,Waters Acquity Ⅰ型高效液相色谱仪和Waters Xevo TQ-S型三重四极杆质谱仪由美国Waters公司生产。CRP、PCT、血常规、乳酸(LAC)等检测均由我院检验中心完成。
1.3 统计学分析采用SPSS 19.0统计软件对数据进行统计学分析,符合正态分布计量资料采用均数±标准差(x±s)表示,两组间比较采用两独立样本t检验;不符合正态分布计量资料采用中位数(四分位间距)[M(P25,P75)]表示,两组间比较采用Mann-Whitney U检验。计数资料采用百分率(%)表示,两组间比较采用卡方检验或校正卡方检验。绘制受试者工作特征曲线(receiver operating characteristic curve, ROC),计算曲线下面积(AUC),ROC评估各指标对脓毒症预后的预测价值。P < 0.05为差异有统计学意义。
2 结果 2.1 脓毒症患儿血浆内生吗啡浓度脓毒症组患儿均检测到血浆内生吗啡,浓度为2.31(0.54, 31.77)ng/mL,16例普通感染组患儿中只有3例可检测到血浆内生吗啡,分别为0.11 ng/mL、0.97 ng/mL、3.89 ng/mL,健康对照组儿童均未检测到血浆内生吗啡。
2.2 脓毒症非休克组和休克组临床资料及血浆内生吗啡浓度根据是否伴有休克将脓毒症患儿分为脓毒症非休克组(n=19)和休克组(n=12)。脓毒症休克组患儿内生吗啡浓度、LAC、病死率和pSOFA明显高于非休克组(P < 0.05),CRP、PCT及PICU住院天数在两组中比较差异无统计学意义(P > 0.05),见表 1。
| 表 1 非休克组和休克组脓毒症患儿血浆内生吗啡浓度及临床资料比较 |
|
|
根据1月内是否死亡将脓毒症患儿分为存活组(n=22)和死亡组(n=9),死亡患儿血浆内生吗啡浓度、pSOFA明显高于存活患儿(P < 0.05),而CRP、PCT、LAC和PICU住院天数在两组间比较差异无统计学意义(P > 0.05)。根据是否伴有MODS将脓毒症患儿分为非MODS组(n=13)和MODS组(n=18)。MODS组血浆内生吗啡浓度、CRP、PCT、LAC和病死率明显高于非MODS组(P < 0.05),而PICU住院天数在两组中比较差异无统计学意义(P > 0.05)。见表 2~3。
| 表 2 存活组和死亡组脓毒症患儿血浆内生吗啡浓度及临床资料比较 |
|
|
| 表 3 非MODS组和MODS组脓毒症患儿血浆内生吗啡浓度及临床资料比较 |
|
|
将两组间比较差异有统计学意义的指标,进一步行ROC分析,AUC越大预测效果越好。内生吗啡、pSOFA对脓毒症患儿休克有预测价值(P < 0.05),AUC分别为0.833和0.989。内生吗啡与pSOFA对脓毒症患儿死亡有预测价值(P < 0.05),AUC分别为0.932和0.871。内生吗啡、CRP、PCT和LAC对脓毒症患儿MODS有预测价值(P < 0.05),AUC分别为0.962、0.731、0.765和0.761。见表 4,图 1~3。
| 表 4 各指标对脓毒症患儿预后的预测价值 |
|
|
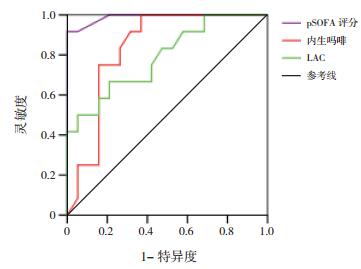
|
图 1 预测脓毒症患儿休克ROC分析图 |
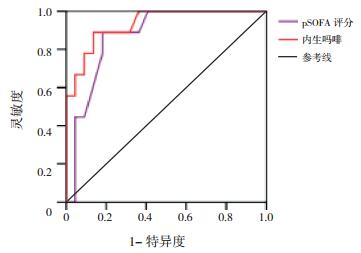
|
图 2 预测脓毒症患儿死亡ROC分析图 |
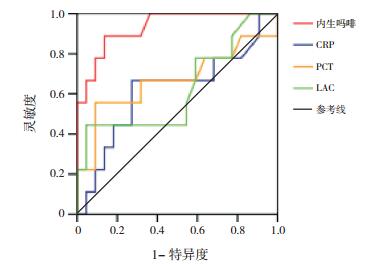
|
图 3 预测脓毒症患儿多器官功能障碍ROC分析图 |
脓毒症时血浆内生吗啡浓度升高可能与机体在严重感染时,刺激免疫细胞产生内生吗啡,继而参与机体的免疫调节机制有关。Glattard等[14]研究发现LPS和IL-8可刺激钙离子依赖的吗啡和乳铁蛋白在体外中性粒细胞分泌;用LPS刺激中性粒细胞后,中性粒细胞表面μ阿片受体表达增加,提示内生吗啡可能是一种信号分子,通过免疫细胞上表达μ阿片受体发挥作用。基于吗啡的免疫抑制作用[17-18],在脓毒症患者中,可能通过增加吗啡水平下调免疫和血管组织反应,防止过度炎症引起器官功能损害;另一方面,吗啡能增强LPS诱导的促炎症因子表达,激活PKCε并刺激Akt通路ERK1/2和iNOS的上游,增强小胶质细胞m-opioid receptor-PKCε通路中IL-1β、TNF-α、IL-6和NO的释放,调节小鼠小胶质细胞的促炎症反应[19]。因此,不能排除内生吗啡也可能在某些细胞中有促炎作用。
本研究发现31例脓毒症患儿血浆内生吗啡浓度显著升高,而健康对照组儿童血浆未能检测到内生吗啡,与Glattard等[14]和Laux-Biehlmann等[16]研究结果一致。而普通感染患儿中只有3例检测到血浆内生吗啡,提示内生吗啡只有在发生严重感染时才会显著升高,内生吗啡可能可用来区分普通感染和脓毒症患儿,作为诊断儿童脓毒症的生物标志物。关于儿童脓毒症血浆内生吗啡浓度目前国内外罕见报道,还需更多的临床研究进一步证实。
本研究31例脓毒症患儿死亡9例,病死率为29%,与国内研究结果相似[20]。休克组内生吗啡浓度、pSOFA、LAC、病死率显著高于非休克组,差异有统计学意义,而两组患儿的血清PCT和CRP水平比较差异无统计学意义,提示内生吗啡预测脓毒症休克优于PCT和CRP,对预后判断有一定的临床价值[21]。死亡患儿血浆内生吗啡浓度、pSOFA明显高于存活患儿,而两组患儿的血清PCT和CRP水平比较差异无统计学意义,提示内生吗啡有可能与脓毒症患儿的病情轻重有关,和pSOFA一样,在一定程度上能反映病情严重程度[22-23]。MODS患儿血浆内生吗啡浓度、CRP、PCT、LAC和病死率明显高于非MODS患儿,ROC结果显示内生吗啡的AUC大于CRP、PCT和LAC。ROC结果显示血浆内生吗啡预测脓毒症患儿休克、死亡及MODS风险AUC值分别为0.833、0.932和0.962,进一步说明血浆内生吗啡对脓毒症患儿的预后有较好的预测价值。综上所述,CRP、PCT预测脓毒症患儿预后的价值低于内生吗啡,在一定程度上提示CRP和PCT作为脓毒症诊断和预后判断的生物标志物受到了挑战。由于脓毒症的临床症状及体征的可变性和非特异性,患儿常同时并发内科或外科情况而混淆诊断,所以早期诊断和区分脓毒症的严重程度具有挑战性,广泛使用的快速反应系统在教学医院中指导医生对脓毒症进行早期诊断和早期干预[24],生物标记物在脓毒症的早期诊断、治疗决策的制订、预后的改善等方面发挥关键性作用[10]。血浆CRP和PCT是脓毒症最常使用的生物标记物。CRP的测量便宜、快速,但手术或外伤后也可以增高,所以特异性差;血清PCT被视为细菌感染的生物标志物,血清PCT在非感染状况下也升高,如外伤、外科手术、胰腺炎和肾功能损害[7, 25],Wacker等[26]对3 000多例拟诊为脓毒症病人进行研究,发现PCT对所有脓毒症患者的可靠诊断缺乏足够的敏感度,阴性时排除脓毒症的特异性也不高,PCT对脓毒症的预测价值有限[27-28]。本研究发现正常儿童检测不到内生吗啡,个别感染患儿仅轻度增高,脓毒症时明显增高,且增高的程度与脓毒症病情严重程度及预后有关,提示内生吗啡有可能作为脓毒症诊断、预后判断的新的生物学指标。
本研究的不足之处在于样本量偏小,且因为临床方面的限制以及大部分脓毒症患儿需使用镇静药,考虑到镇静药可能会影响血浆内生吗啡浓度,遂未按时间点收集脓毒症血标本,内生吗啡是否能作为判断脓毒症的生物标志物及预后判断指标还需要多样本、多中心、多时间点的临床研究。
| [1] |
中华医学会儿科学分会急救学组, 中华医学会急诊医学分会儿科学组, 中国医师协会儿童重症医师分会. 儿童脓毒性休克(感染性休克)诊治专家共识(2015版)[J]. 中华儿科杂志, 2015, 53(8): 576-580. (  0) 0) |
| [2] |
Lagu T, Rothberg MB, Shieh MS, et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007[J]. Crit Care Med, 2012, 40(3): 754-761. DOI:10.1097/CCM.0b013e318232db65 (  0) 0) |
| [3] |
Liu Y, Yang W, Wei J, et al. Guiding effect of serum procalcitonin (PCT) on the antibiotic application to patients with sepsis[J]. Iran J Public Health, 2017, 46(11): 1535-1539. (  0) 0) |
| [4] |
Ivaska L, Elenius V, Mononen I, et al. Discrepancies between plasma procalcitonin and C-reactive protein levels are common in acute illness[J]. Acta Paediatr, 2016, 105(5): 508-513. DOI:10.1111/apa.13293 (  0) 0) |
| [5] |
Jämsä J, Ala-Kokko T, Huotari V, et al. Neutrophil CD64, C-reactive protein, and procalcitonin in the identification of sepsis in the ICU-post-test probabilities[J]. J Crit Care, 2018, 43: 139-142. DOI:10.1016/j.jcrc.2017.08.038 (  0) 0) |
| [6] |
Pontrelli G, De Crescenzo F, Buzzetti R, et al. Accuracy of serum procalcitonin for the diagnosis of sepsis in neonates and children with systemic inflammatory syndrome:a meta-analysis[J]. BMC Infect Dis, 2017, 17(1): 302. DOI:10.1186/s12879-017-2396-7 (  0) 0) |
| [7] |
Hoshino K, Irie Y, Mizunuma M, et al. Incidence of elevated procalcitonin and presepsin levels after severe trauma:a pilot cohort study[J]. Anaesth Intensive Care, 2017, 45(5): 600-604. (  0) 0) |
| [8] |
Annborn M, Dankiewicz J, Erlinge D, et al. Procalcitonin after cardiac arrest-an indicator of severityof illness, ischemia-reperfusion injury and outcome[J]. Resuscitation, 2013, 84(6): 782-787. DOI:10.1016/j.resuscitation.2013.01.004 (  0) 0) |
| [9] |
Franeková J, Sečník P Jr, Lavríková P, et al. Serial measurement of presepsin, procalcitonin, and C-reactive protein in the early postoperative period and the response to antithymocyte globulin administration after heart transplantation[J]. Clin Transplant, 2017, 31: e12870. DOI:10.1111/ctr.2017.31.issue-1 (  0) 0) |
| [10] |
Brodska H, Valenta J, Pelinkova K, et al. Diagnostic and prognostic value of presepsin vs. established biomarkers in critically ill patients with sepsis or systemic inflammatory response syndrome[J]. Clin Chem Lab Med, 2018, 56(4): 658-668. DOI:10.1515/cclm-2017-0839 (  0) 0) |
| [11] |
van Engelen TSR, Wiersinga WJ, Scicluna BP, et al. Biomarkers in sepsis[J]. Crit Care Clin, 2018, 34(1): 139-152. DOI:10.1016/j.ccc.2017.08.010 (  0) 0) |
| [12] |
Bruehl S, Burns JW, Gupta R, et al. Endogenous opioid inhibition of chronic low-back pain influences degree of back pain relief after morphine administration[J]. Reg Anesth Pain Med, 2014, 39(2): 120-125. DOI:10.1097/AAP.0000000000000058 (  0) 0) |
| [13] |
Fan Y, Liang X, Wang R, et al. Role of endogenous melatoninergic system in development of hyperalgesia and tolerance induced by chronic morphine administration in rats[J]. Brain Res Bull, 2017, 135: 105-112. DOI:10.1016/j.brainresbull.2017.10.005 (  0) 0) |
| [14] |
Glattard E, Welters ID, Lavaux T, et al. Endogenous morphine levels are increased in sepsis:a partial implication of neutrophils[J]. PLoS One, 2010, 5(1): e8791. DOI:10.1371/journal.pone.0008791 (  0) 0) |
| [15] |
Matics TJ, Sanchez-Pinto LN. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the sepsis-3 definitions in critically ill children[J]. JAMA Pediatr, 2017, 171(10): e172352. DOI:10.1001/jamapediatrics.2017.2352 (  0) 0) |
| [16] |
Laux-Biehlmann A, Gräfe N, Mouheiche J, et al. Comparison of serum and lithium-heparinate plasma for the accurate measurements of endogenous and exogenous morphine concentrations[J]. Br J Clin Pharmacol, 2012, 74(2): 381-383. DOI:10.1111/j.1365-2125.2012.04199.x (  0) 0) |
| [17] |
Mao M, Qian Y, Sun J. Morphine suppresses T helper lymphocyte differentiation to Th1 type through PI3K/AKT pathway[J]. Inflammation, 2016, 39(2): 813-821. DOI:10.1007/s10753-016-0310-x (  0) 0) |
| [18] |
Wan J, Ma J, Anand V, et al. Morphine potentiates LPS-induced autophagy initiation but inhibits autophagosomal maturation through distinct TLR4-dependent and independent pathways[J]. Acta Physiol (Oxf), 2015, 214(2): 189-199. DOI:10.1111/apha.2015.214.issue-2 (  0) 0) |
| [19] |
Merighi S, Gessi S, Varani K, et al. Morphine mediates a proinflammatory phenotype via μ-opioid receptor-PKCε-Akt-ERK1/2 signaling pathway in activated microglial cells[J]. Biochem Pharmacol, 2013, 86(4): 487-496. DOI:10.1016/j.bcp.2013.05.027 (  0) 0) |
| [20] |
吴忠瑾, 陈亚凤, 王海岛, 等. 血浆miRNA-497在小儿脓毒症心肌损伤中的表达及其临床意义[J]. 中国当代儿科杂志, 2018, 20(1): 32-36. DOI:10.7499/j.issn.1008-8830.2018.01.007 (  0) 0) |
| [21] |
Solligård E, Damås JK. SOFA criteria predict infection-related in-hospital mortality in ICU patients better than SIRS criteria and the qSOFA score[J]. Evid Based Med, 2017, 22(6): 211. DOI:10.1136/ebmed-2017-110727 (  0) 0) |
| [22] |
Hamshary AAEE, Sherbini SAE, Elgebaly HF, et al. Prevalence of multiple organ dysfunction in the pediatric intensive care unit:pediatric risk of mortality Ⅲ versus pediatric logistic organ dysfunction scores for mortality prediction[J]. Rev Bras Ter Intensiva, 2017, 29(2): 206-212. (  0) 0) |
| [23] |
Fukushima H, Kobayashi M, Kawano K, et al. Performance of quick sequential (sepsis related) and sequential (sepsis related) organ failure assessment to predict mortality in patients with acute pyelonephritis associated with upper urinary tract calculi[J]. J Urol, 2018, 199(6): 1526-1533. DOI:10.1016/j.juro.2017.12.052 (  0) 0) |
| [24] |
Cross G, Bilgrami I, Eastwood G, et al. The epidemiology of sepsis during rapid response team reviews in a teaching hospital[J]. Anaesth Intensive Care, 2015, 43(2): 193-198. (  0) 0) |
| [25] |
Dai X, Fu C, Wang C, et al. The impact of tracheotomy on levels of procalcitonin in patients without sepsis:a prospective study[J]. Clinics (Sao Paulo), 2015, 70(9): 612-617. DOI:10.6061/clinics (  0) 0) |
| [26] |
Wacker C, Prkno A, Brunkhorst FM, et al. Procalcitonin as a diagnostic marker for sepsis:a systematic review and meta-analysis[J]. Lancet Infect Dis, 2013, 13(5): 426-435. DOI:10.1016/S1473-3099(12)70323-7 (  0) 0) |
| [27] |
Angeletti S, Dicuonzo G, Fioravanti M, et al. Procalcitonin, MR-proadrenomedullin, and cytokines measurement in sepsis diagnosis:advantages from test combination[J]. Dis Markers, 2015, 2015: 951532. (  0) 0) |
| [28] |
Trippella G, Galli L, De Martino M, et al. Procalcitonin performance in detecting serious and invasive bacterial infections in children with fever without apparent source:a systematic review and meta-analysis[J]. Expert Rev Anti Infect Ther, 2017, 15(11): 1041-1057. DOI:10.1080/14787210.2017.1400907 (  0) 0) |
 2018, Vol. 20
2018, Vol. 20


