2. Department of Pathology, Faculty of Basic Science and Paraclinical Science, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
Obstructive jaundice in children beyond the neonatal period can be caused by choledochal cyst, congenital biliary tract abnormalities, gallstones, strictures due to chronic cholangitis and uncommonly by neoplastic biliary obstruction due to rhabdomyosarcoma (RMS)[1-2]. RMS, a skeletal muscle malignancy, is the soft tissue tumour most common among children aged 0-14 years, accounting for 1% of all malignancies[1, 3-4]. Median age is about 3 years for the occurrence of hepatobiliary RMS in children[1]. This uncommon tumour was originally reported by Wilks and Moxon in 1875[5]. Because of its low incidence, diagnosis of biliary RMS is very challenging, needing a high degree of suspicion and the use of new and appropriate imaging techniques[6]. As embryonal RMS is both solid and cystic, preoperative imaging may not suffice to define the lesion exactly[7]. Due to newer radiological techniques and available combined therapy modalities, treatment and prognosis of biliary RMS are frequently changing[8].
2 Case reportA 10-year-old boy, the only child of non-consanguineous parents, immunized according to the Expanded Programme on Immunization (EPI) schedule, presented to the Paediatric Gastroenterology and Nutrition Department, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh with high-grade intermittent fever, occasionally associated with chills, rigors and jaundice, accompanying by anorexia, but no nausea and vomiting. He had occasional abdominal pain, mild and diffuse, involving mostly the right upper abdomen. There was a history of similar attack two months back. He had no history of pruritus, coagulopathy or encephalopathy. On examination, he was conscious, oriented, cooperative, mildly pale, icteric and febrile, with stable vital signs, no stigmata of chronic liver disease and having normal growth. Systemic examination revealed a soft abdomen, no palpable lump, nontender just palpable liver. And absence of splenomegaly, ascites, skin rash or cervical lymphadenopathy.
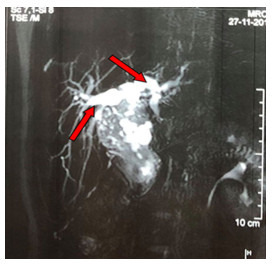
There was a neutrophilic leucocytosis with a high erythrocyte sedimentation rate. The liver function tests were abnormal. Regarding viral markers HBsAg, anti-HAV IgM, anti-HCV IgM were negative except antibodies to herpes simplex virus type 1 (anti-HSV1) IgM were positive (Table 1). In imaging findings, ultrasonogram of the abdomen showed slightly enlarged liver with homogenous echotexture, slightly dilated intrahepatic biliary channels, and fusiform dilatation (3.8 cm × 5.2 cm × 5.8 cm) of the common bile duct (CBD) with soft tissue extending into the distal part of CBD compressing the head of the pancreas, which is suggestive of a choledochal cyst (infected or hemorrhagic – type IVb). An irregular hypoechoic soft tissue mass (5.3 cm × 3.8 cm) near the head of the pancreas and flow within the mass with Resistance Index (RI) = 0.51 is seen in the Doppler study of the portal vein, hepatic veins, and inferior vena cava. Magnetic resonance cholangiopancreatogram (MRCP) revealed gross fusiform dilatation of CBD and common hepatic duct (CHD) producing a sac-like mass 4.3 cm × 2.7 cm in size, slightly dilated intrahepatic biliary channels, and low signal intensity within the dilated CBD and these features suggestive of a choledochal cyst (Type IVb) with sludge and secondary dilatation of intrahepatic biliary radicles (Figure 1).
| Table 1 Laboratory indexes |
|
|
|
Figure 1 Results of coronal magnetic resonance cholangiopancreatogram Gross fusiform dilation of the CBD (red arrow) and slightly dilated intrahepatic biliary channel. |
Intraoperatively, the CBD was widely dilated, and its wall was thin and oedematous. The cystic dilatation of the CBD contained soft tissue, necrotic and tumorous tissue up to intrahepatic bile duct (Figure 2).

|
Figure 2 Intraoperative findings of the CBD Soft tissue, necrotic tissue, and tumorous tissue are observed in the CBD. |
Choledochotomy was done. Soft, necrotic and tumorous tissue was cleared off as far as possible from the CBD and sent for histopathology. Proximal and distal patency of the CBD was ensured and a T-tube placed in situ before closure. No evidence of metastasis or lymph node involvement was found.
Histopathology report was suggestive of malignant small round blue cell tumour (Figure 3A) followed by immunohistochemistry revealed tumour cells were positive for desmin, myogenin and vimentin whereas negative for pancytokeratin and leukocyte common antigen which were compatible with embryonal rhabdomyosarcoma (Figure 3 B-D).

|
Figure 3 Results of histopathology A: Perivascular condensations of malignant cells have hyperchromatic round nuclei and contain generous amounts of eosinophilic cytoplasm. The stroma is loose and myxoid (red arrow, Hematoxylin and Eosin, ×200). B: Tumor cells and stroma show diffuse positive immunoreactivity (red arrow, Vimentin, ×100). C: Tumor cells show diffuse positive immunoreactivity (red arrow, Desmin, ×100). D: Most of the tumor cells show positive immunoreactivity (red arrow, Myogenin, ×100). |
Postoperatively, patient received vincristine, adriamycin and cyclophosphamide (VAC) chemotherapy. There were no apparent distant metastasis, as chest X-ray and bone marrow study of the patient were normal. During three months of follow up, there were no findings of recurrence, and the obstructive jaundice resolved. Close follow up is being continued.
3 DiscussionCommon causes of obstructive jaundice in children include hepatitis, drug-induced jaundice, and benign, recurrent cholestasis[9]. Surgical causes of obstructive jaundice include choledochal cysts, choledocholithiasis, strictures, congenital anomalies, and rarely RMS[6]. For the exclusion of this etiology, the first investigation should be ultrasonogram. In this case, the ultrasound scan demonstrated fusiform dilatation of the CBD with soft tissue structure resultant obstructive jaundice. Congenital anomalies like stricture and choledocholithiasis were excluded by these initial imaging findings leaving the imaging differential diagnosis as choledochal cyst filled with sludge and primary biliary neoplasm or primary liver tumor with metastasis to the biliary tree. However, the hepatic neoplasm was excluded due to normal hepatic architecture.
Histological subtypes of RMS are embryonal, botryoid, alveolar, spindle cell variant, and RMS not otherwise specified[10]. Among them, the biliary tract has neoplasms of only embryonal or botryoid histology. Embryonal variety is three times more common than other varieties but the botryoid subtype has the best prognosis[11]. Embryonal RMS in children mainly presents in the head and neck, genitourinary tract, extremities, parameningeal area, retroperitoneum, and rarely biliary tract[12]. It may arise in any part of the biliary channel[13] or within a choledochal cyst[14]. In this case, RMS occurred in the wall of the CBD. RMS of the biliary tree is a disease of young children, 1-9 years of age with a slight male predominance[10, 15-16].
RMS presents with obstructive jaundice in 60%-80% of cases[17]. Other less common presenting features are abdominal pain, abdominal distension, anorexia, nausea, vomiting, and fever[6, 16, 18-19]. In this case, the patient presented with recurrent obstructive jaundice, fever, abdominal pain, anorexia, and hepatomegaly. Conjugated hyperbilirubinemia and raised liver enzymes are commonly seen as in our case. In our case, leucocytosis was due to cholangitis.
The initial imaging modality for diagnosis should be the ultrasonogram. Ultrasonogram with color Doppler is the optimal method of assessing the response to preoperative and postoperative chemotherapy[6]. The characteristic sonographic finding of biliary RMS is a biliary system filled with solid cystic content giving a lace-like appearance and the presence of intralesional vascularity by color Doppler confirms the possibility[20-21]. A preoperative sonogram can show biliary cystic dilatation, which may suggest a congenital choledochal cyst. Because of bile stasis and filling of the lumen by soft, inspissated debris, both solid and cystic areas may present within an embryonal RMS and for that reason, an embryonal RMS can mimic the radiological features of a choledochal cyst that occurred in our case[7, 22-23]. Embryonal RMS may occur within a choledochal cyst in the literature but histopathologically it is not possible to confirm, whether it is primary RMS of the CBD or within the choledochal cyst[24].
For better evaluation of the biliary channel, we did MRCP that revealed gross fusiform dilatation of the CBD producing sac-like mass and intrahepatic biliary channels slightly dilated, suggestive of choledochal cyst with sludge and secondary dilatation of intrahepatic biliary radicles. Other imaging techniques may be computed tomography (CT) scan or magnetic resonance imaging (MRI). The identification of the solid nature of the tumor by CT scan can sometimes aid differentiation from a choledochal cyst[19]. CT scan and MRI are also helpful to see the extent and staging of the disease[6].
Embryonal RMS is usually confirmed postoperatively using histology and immuno-histochemistry. Myoblastic differentiation and expression of skeletal muscle markers, such as desmin, myogenin, and/or myoD1 found in RMS by histopathology[25]. Over 90% of embryonal RMS are positive for desmin[26], but this is non-specific. Recently it was shown that myogenin is a specific immune-histochemical marker for embryonal RMS[17, 26]. In our case, histopathology was positive for malignant small round blue cell tumor and immune-histochemical staining was positive for desmin, vimentin, and myogenin.
Over the past 30 years, embryonal RMS therapy has greatly improved and the cure rate increased 3-fold[27]. Surgical removal, chemotherapy, and radiotherapy are the current modalities of treatment[27]. The Intergroup Rhabdomyosarcoma Study IV recommends a combination of vincristine, actinomycin D and cyclophosphamide, ifosfamide, or etoposide as chemotherapy for embryonal RMS[12]. Besides, most children with embryonal RMS also need surgery and/or radiotherapy to achieve local tumor control[17]. In our case, after surgical resection, VAC chemotherapy is being given. Although gross total excision is not always possible, the prognosis remains relatively good which is probably owing to the favorable histology. Neoadjuvant chemotherapy followed by resection of the residual tumor has been associated with good outcomes[25]. The absence of distant metastasis, embryonal variety, and small size ≤5 cm, place our patient in the intermediate-risk group according to Children's Oncology Group[10].
In conclusion, biliary RMS presents with obstructive jaundice and in cystic tumors with central necrosis can mimic a choledochal cyst. Thus, embryonal RMS should be considered in the differential diagnosis of children presenting with obstructive jaundice and a cystic or soft tissue mass within the CBD.
Acknowledgments:Professor Mohsen Chowdhury provided valuable information regarding the surgical perspective and Professor Afiqul Islam provided the chemotherapy perspective for this case.
| [1] |
Ali S, Russo MA, Margraf L. Biliary rhabdomyoscarcoma mimicking choledochal cyst[J]. J Gastrointestin Liver Dis, 2009, 18(1): 95-97. (  0) 0) |
| [2] |
Raina S, Kumar N, Jad B, et al. Botryoid rhabdomyosarcoma of biliary tree:a diagnostic puzzle[J]. IOSR-JDMS, 2015, 14(8): 94-96. (  0) 0) |
| [3] |
Zampieri N, Camoglio F, Corroppolo M, et al. Botryoid rhabdomyosarcoma of the biliary tract in children:a unique case report[J]. Eur J Cancer Care (Engl), 2006, 15(5): 463-466. DOI:10.1111/j.1365-2354.2006.00683.x (  0) 0) |
| [4] |
Enzinger FM, Weiss SW. Rhabdomyosarcoma[M]//Enzinger FM, Weiss SW. Soft tissue tumours. 4th ed. St Louis, Missouri: Mosby, 2001: 790.
(  0) 0) |
| [5] |
Wilks S, Moxon W. Lectures on Pathological Anatomy[M]. 2nd ed. Philadelphia: Lindsay and Blakiston: 465-466.
(  0) 0) |
| [6] |
Kinariwala DJ, Wang AY, Melmer PD, et al. Embryonal rhabdomyosarcoma of the biliary tree:a rare cause of obstructive jaundice in children which can mimic choledochal cysts[J]. Indian J Radiol Imaging, 2017, 27(3): 306-309. DOI:10.4103/ijri.IJRI_460_16 (  0) 0) |
| [7] |
Tireli GA, Sander S, Dervisoglu S, et al. Embryonal rhabdomyosarcoma of the common bile duct mimicking choledochal cyst[J]. J Hepatobiliary Pancreat Surg, 2005, 12(3): 263-265. DOI:10.1007/s00534-004-0959-7 (  0) 0) |
| [8] |
Lad SK, Balasubramanian M, Kavishwar V, et al. Botryoid rhabdomyosarcoma of the common bile duct[J]. Bombay Hosp J, 2010, 52: 108-110. (  0) 0) |
| [9] |
Ruymann FB, Takeuchi A, Boyce HW. Idiopathic, recurrent cholestasis[J]. Pediatrics, 1970, 45(5): 812-820. (  0) 0) |
| [10] |
Levy CF, Wexler LH. Rhabdomyosarcoma and Other Soft-Tissue Sarcomas[M]//Lanzkowsky P, Lipton JM, Fish JD. Lanzkowsky's Manual of Pediatric Hematology and Oncology. 6th ed. USA: Academic Press, 2016: 505-523.
(  0) 0) |
| [11] |
Parham DM. Pathologic classification of rhabdomyosarcomas and correlations with molecular studies[J]. Mod Pathol, 2001, 14(5): 506-514. DOI:10.1038/modpathol.3880339 (  0) 0) |
| [12] |
Baker KS, Anderson JR, Link MP, et al. Benefit of intensified therapy for patients with local or regional embryonal rhabdomyosarcoma:results from the Intergroup Rhabdomyosarcoma Study IV[J]. J Clin Oncol, 2000, 18(12): 2427-2434. DOI:10.1200/JCO.2000.18.12.2427 (  0) 0) |
| [13] |
Crist WM, Anderson JR, Meza JL, et al. Intergroup rhabdomyosarcoma study-IV:results for patients with nonmetastatic disease[J]. J Clin Oncol, 2001, 19(12): 3091-3102. DOI:10.1200/JCO.2001.19.12.3091 (  0) 0) |
| [14] |
Arnaud O, Boscq M, Asquier E, et al. Embryonal rhabdomyosarcoma of the biliary tree in children:a case report[J]. Pediatr Radiol, 1987, 17(3): 250-251. DOI:10.1007/BF02388175 (  0) 0) |
| [15] |
Mathew M, Narula MK, Chadha R. Case report:sarcoma botryoides of the common bile duct[J]. Indian J Radiol Imaging, 2001, 11: 101-102. (  0) 0) |
| [16] |
Sanz N, de Mingo L, Florez F, et al. Rhabdomyosarcoma of the biliary tree[J]. Pediatr Surg Int, 1997, 12(2-3): 200-201. DOI:10.1007/BF01350002 (  0) 0) |
| [17] |
Nemade B, Talapatra K, Shet T, et al. Embryonal rhabdomyosarcoma of the biliary tree mimicking a choledochal cyst[J]. J Cancer Res Ther, 2007, 3(1): 40-42. (  0) 0) |
| [18] |
Spunt SL, Lobe TE, Pappo AS, et al. Aggressive surgery is unwarranted for biliary tract rhabdomyosarcoma[J]. J Pediatr Surg, 2000, 35(2): 309-316. DOI:10.1016/S0022-3468(00)90030-7 (  0) 0) |
| [19] |
Kumar V, Chaudhary S, Kumar M, et al. Rhabdomyosarcoma of biliary tract-a diagnostic dilemma[J]. Indian J Surg Oncol, 2012, 3(4): 314-316. DOI:10.1007/s13193-012-0186-7 (  0) 0) |
| [20] |
Sharma AK, Lahoti BK, Aggarwal R, et al. Obstructive jaundice in children may be due to a malignant tumour of the common bile duct[J]. Pediatr Surg Int, 1995, 10(7): 496-497. (  0) 0) |
| [21] |
Majmudar B, Kumar VS. Embryonal rhabdomyosarcoma (sarcoma botryoides) of the common bile duct:a case report[J]. Hum Pathol, 1976, 7(6): 705-708. DOI:10.1016/S0046-8177(76)80081-0 (  0) 0) |
| [22] |
Davis GL, Kissane JM, Ishak KG. Embryonal rhabdomy-osarcoma (sarcoma botryoides) of the biliary tree. Report of five cases and a review of the literature[J]. Cancer, 1969, 24(2): 333-342. (  0) 0) |
| [23] |
Verstandig A, Bar-Ziv J, Abu-Dalu KI, et al. Sarcoma botryoides of the common bile duct:preoperative diagnosis by coronal CT and PTC[J]. Pediatr Radiol, 1991, 21(2): 152-153. DOI:10.1007/BF02015637 (  0) 0) |
| [24] |
Patil KK, Omojola MF, Khurana P, et al. Embryonal rhabdomyosarcoma within a choledochal cyst[J]. Can Assoc Radiol J, 1992, 43(2): 145-148. (  0) 0) |
| [25] |
Mathew D, de Lima H, Mahomed N. Embryonal rhabdomyosarcoma of the biliary tree in a paediatric patient-a rare cause of obstructive jaundice[J]. SA J Radiol, 2019, 23(1): 1662. (  0) 0) |
| [26] |
Morotti RA, Nicol KK, Parham DM, et al. An immuno-histochemical algorithm to facilitate diagnosis and subtyping of rhabdomyosarcoma:the Children's Oncology Group experience[J]. Am J Surg Pathol, 2006, 30(8): 962-968. DOI:10.1097/00000478-200608000-00005 (  0) 0) |
| [27] |
Breneman JC, Lyden E, Pappo AS, et al. Prognostic factors and clinical outcomes in children and adolescents with metastatic rhabdomyosarcoma-a report from the Intergroup Rhabdomyosarcoma Study IV[J]. J Clin Oncol, 2003, 21(1): 78-84. DOI:10.1200/JCO.2003.06.129 (  0) 0) |
 2020, Vol. 22
2020, Vol. 22

