随着化疗方案的改进,儿童急性淋巴细胞白血病(acute lymphoblastic leukemia, ALL)的5年无事件生存率(event-free survival, EFS)已高达90%,而接受规范的维持治疗是基础[1-3]。6-巯基嘌呤(6-mercaptopurine, 6-MP)是ALL维持治疗的重要药物,它通过抑制白血病细胞DNA和RNA合成从而影响嘌呤核苷酸合成而发挥抗癌作用。但6-MP治疗窗窄,最小有效剂量至中毒剂量的范围小,药代学个体差异大。因此,6-MP个体化治疗成为研究热点。研究[4]发现硫代嘌呤甲基转移酶(thiopurine S-methyltransferase, TPMT)参与6-MP代谢物的甲基化,其活性缺乏与6-MP不耐受具有相关性。但我国TPMT的等位基因突变频率低于欧美国家,并且一些具有正常TPMT活性的患者仍然可能发生6-MP相关的毒副作用,因此单纯以TPMT多态性不能完全解释硫嘌呤相关毒性的个体差异[5-6]。近来发现具有NUDT15基因突变的ALL患者也不能耐受6-MP常规剂量[7-10]。本文就NUDT15基因型对儿童ALL患者6-MP个体化治疗的影响进行综述。
1 6-MP代谢过程以及NUDT15基因突变对6-MP代谢的影响机制6-MP需代谢转化为相应的活性化合物才能发挥作用,代谢过程见图 1。6-MP由一系列酶催化最终产生的6-巯基鸟嘌呤三磷酸盐(6-TGTP)及6-脱氧巯基鸟嘌呤三磷酸盐(6-dTGTP)分别被并入RNA(RNA-TG)和DNA(DNA-TG),通过错配修复导致细胞周期停滞和细胞凋亡,使DNA链断裂而产生细胞毒性[11-12]。NUDT15是一种水解酶,属于nudix(nucleoside diphosphate linked moiety X)蛋白,能催化各种生化反应[13]。在6-MP代谢过程中,NUDT15主要参与6-硫基次黄嘌呤单磷酸盐(6-TIMP)向三磷酸盐(6-TGTP、6-dTGTP)转化的逆反应过程,阻止6-dTGTP并入DNA,从而使DNA损伤最小化并避免细胞凋亡。
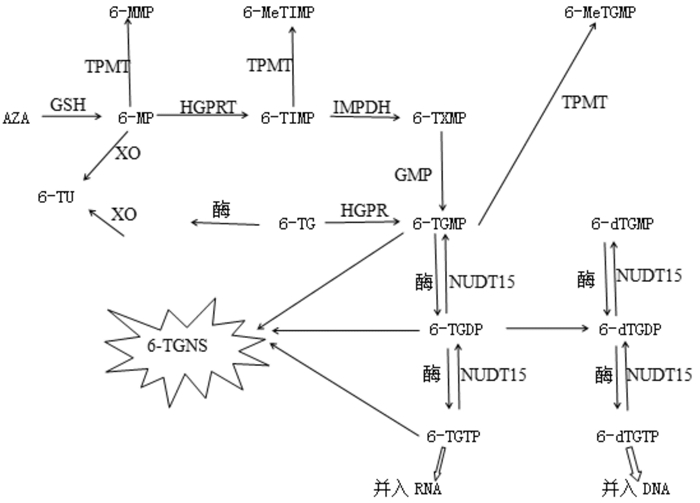
|
图 1 -MP代谢过程 [AZA]硫唑嘌呤;[GSH]谷胱甘肽;[GMPS]鸟嘌呤核苷一磷酸脱氢酶;[HPRT]次黄嘌呤磷酸核糖转移酶;[IMPDH]次黄嘌呤单磷酸脱氢酶;[6-MMP] 6-甲基硫基嘌呤;[6-MeTIMP] 6-甲基硫次黄嘌呤核苷一磷酸;[6-MeTGMP] 6-甲基硫鸟嘌呤核苷一磷酸;[6-dTGMP] 6-脱氧巯基鸟嘌呤单磷酸盐;[6-dTGDP] 6-脱氧巯基鸟嘌呤二磷酸盐;[6-dTGTP] 6-脱氧巯基鸟嘌呤三磷酸盐;[6-TGNS] 6-硫鸟苷酸;[6-TGMP] 6-巯基鸟嘌呤单磷酸盐;[6-TGDP] 6-巯基鸟嘌呤二磷酸盐;[6-TGTP] 6-巯基鸟嘌呤三磷酸盐;[6-TIMP] 6-硫基次黄嘌呤单磷酸盐;[6-TU] 6-硫尿酸;[6-TXMP] 6-硫基黄嘌呤单磷酸盐;[XO]黄嘌呤氧化酶。 |
NUDT15基因突变导致NUDT15酶活性降低,6-TIMP向三磷酸盐转化的逆反应过程受到影响,6-TGTP、6-dTGTP水平增高,从而巯嘌呤的细胞毒性增强[14]。但Asada等[15]分析161例接受6-MP治疗的炎性肠病患者发现,白细胞减少的45例患者中不同NUDT15基因型患者的6-TGN水平没有显著差异。也有学者[16]认为,NUDT15基因可能通过编码核苷二磷酸酶,使氧化嘌呤核苷三磷酸(例如8-氧代-dGTP)去磷酸化,以防止其损伤细胞。因此,NUDT15基因突变对6-MP代谢影响的机制还有待进一步研究。
2 NUDT15基因多态性目前报道[17]的NUDT15等位基因及其突变基因共有6种,分别命名为NUDT15*1至NUDT15*6,突变位点及对应氨基酸变异的蛋白质见表 1。此外,在亚裔、非洲或欧洲血统的儿童中发现3种表 1以外的新型变体:p.R34T,p.K35E,p.G17_V18del [18]。
| 表 1 NUDT15等位基因突变 |
|
|
理论上6种NUDT15等位基因突变单倍型共有21种基因型,即野生型(C/C)、杂合型(C/T)及纯合型(T/T),见表 2[19]。野生型表现高等活性;杂合型即等摩尔NUDT15基因野生型与突变型编码蛋白的混合物,显示中等活性;纯合型表现为低等活性,包括双突变纯合型、双突变杂合型[20-21]。
| 表 2 NUDT15基因型及其活性 |
|
|
研究[22]表明NUDT15(rs116855232)基因变异率在东亚人群最高(9.8%),其次是西班牙(3.9%),欧洲人群仅为0.2%,而在非洲没有发现。Zhang等[23]对1 138名ALL、炎性肠病患者的荟萃分析显示,在亚洲NUDT15基因突变C.415C > T的等位基因(C和T)出现频率分别为86.72%和13.28%。Moriyama等[19]在270例来自危地马拉、新加坡、日本的ALL儿童中发现4种NUDT15基因突变:p.Arg139Cys、p.Arg139His、p.Val18Ile和p.Val18_Val19insGlyVal。日本、韩国、乌圭拉、黎巴嫩等多个地区均发现NUDT15基因突变,其对应变异率(包括CC和CT)约为20%、40%、17%、1%[24-27]。
目前我国对NUDT15基因突变的研究相对较少。Liang[28]在304例急淋患儿和100例克罗恩病患儿中发现22.0%存在NUDT15基因突变。中山大学[29]对253名克罗恩病的研究发现,中国人群NUDT15基因突变率约为22.5%(CT 20.9% vs TT 1.6%)。这些研究提示亚洲国家NUDT15基因突变频率较高[22]。
4 NUDT15基因型对6-MP个体化治疗的影响研究[30-32]发现,接受6-MP常规剂量治疗的ALL患者中存在NUDT15基因纯合突变的骨髓抑制最严重,杂合突变患者有中度至重度骨髓抑制,而NUDT15基因野生型的患者骨髓抑制最轻。
研究[28-29]表明:中国台湾和日本的NUDT15突变基因杂合型(CT)患者的6-MP耐受剂量为野生型的70%左右,而韩国NUDT15基因杂合型(CT)患者6-MP剂量范围为20%~80%,中国台湾、日本及韩国NUDT15突变基因纯合型(TT)患者的6-MP耐受剂量为野生型的25%。此外,有研究[33]指出年龄、6-TGN水平也与6-MP耐受性密切相关。因此,关于具有NUDT15基因突变ALL患者的6-MP剂量原则还需要进一步大样本多中心研究。
研究还发现一些具有NUDT15基因突变患儿同时伴有TPMT基因突变。Lee等[34]研究显示,3例具有NUDT15基因纯合突变的克罗恩病患儿同时具有TPMT基因突变杂合型,不耐受6-MP治疗。但与之相反的是,日本学者[35]对92名ALL患者的研究显示,NUDT15基因型相同的患儿TPMT基因突变与维持治疗期间6-MP耐受剂量及毒性无关。因此TPMT基因突变与NUDT15基因突变对于6-MP不耐受是否具有协同作用尚未明确。
5 展望综上所述,NUDT15基因突变与ALL患者6-MP耐受性具有相关性,尤其是亚洲人群。关于ALL患儿6-MP个体化治疗尚需要关注以下几个方面:第一、地区、种族对NUDT15基因多态性的影响。第二、TPMT基因突变与NUDT15基因突变对于6-MP耐受性的影响是否具有协同作用。第三、其他基因如如编码三磷酸腺苷结合转运家族蛋白4(ABCC4)、脱嘌呤/脱嘧啶核酸内切酶1(APEX1)等基因多态性是否与6-MP耐受性相关。
| [1] |
Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children[J]. N Engl J Med, 2015, 373: 1541-1552. DOI:10.1056/NEJMra1400972 (  0) 0) |
| [2] |
Pui CH, Yang JJ, Hunger SP, et al. Childhood acute lymphoblastic leukemia:Progress through collaboration[J]. J Clin Oncol, 2015, 33(27): 2938-2948. DOI:10.1200/JCO.2014.59.1636 (  0) 0) |
| [3] |
于洁. 儿童急性淋巴细胞白血病维持治疗的发展和研究[J]. 儿科药学志, 2009, 15(2): 3-6. (  0) 0) |
| [4] |
Lennard L, Cartwright CS, Wade R, et al. Thiopurine methyltransferase and treatment outcome in the UK acute lymphoblastic leukaemia trial ALL2003[J]. British J Haematol, 2015, 170(4): 550-558. DOI:10.1111/bjh.2015.170.issue-4 (  0) 0) |
| [5] |
Maxwell RR, Cole PD. Pharmacogenetic predictors of treatment-related toxicity amongchildren with acute lymphoblastic leukemia[J]. Current Haematol Malig Rep, 2017, 12(3): 176-186. DOI:10.1007/s11899-017-0376-z (  0) 0) |
| [6] |
Yi ES, Choi YB, Choi R, et al. NUDT15 variants cause hematopoietic toxicity with low 6-TGN levels in children with acute lymphoblastic leukemia[J]. Cancer Res Treat, 2018, 50(3): 872-882. DOI:10.4143/crt.2017.283 (  0) 0) |
| [7] |
Singh M, Bhatia P, Khera S. Emerging role of NUDT15 polymorphisms in 6-mercaptopurine metabolism and dose related toxicity in acute lymphoblastic leukaemia[J]. Leuk Res, 2017, 62: 17-22. DOI:10.1016/j.leukres.2017.09.012 (  0) 0) |
| [8] |
Zhu Y, Yin D, Su Y, et al. Combination of common and novel rare variants improves predictive sensitivity of thiopurine induced leukopenia in children with acute lymphoblastic leukemia[J]. Haematologica, 2018, 103(7): e293-e295. DOI:10.3324/haematol.2018.187658 (  0) 0) |
| [9] |
Shah SA, Paradkar M, Desai D. Nucleoside diphosphatelinked moiety X-type motif 15 C415T variant as a predictor for thiopurine-induced toxicity in Indian patients[J]. J Gastroenterol Hepatol, 2017, 32(3): 620-624. DOI:10.1111/jgh.2017.32.issue-3 (  0) 0) |
| [10] |
Yang JJ, Landier W, Yang W, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia[J]. J Clin Oncol, 2015, 33(11): 1235-1242. DOI:10.1200/JCO.2014.59.4671 (  0) 0) |
| [11] |
李志玲, 王鹤尧, 孙华君. 巯嘌呤类药物用于儿童急性淋巴细胞性白血病患者个体化治疗的研究进展[J]. 上海医药, 2015, 36(19): 12-15. (  0) 0) |
| [12] |
Karran P, Attard N. Thiopurines in current medical practice:molecular mechanisms and contributions to therapy-related cancer[J]. Nat Rev Cancer, 2008, 8(1): 24-36. DOI:10.1038/nrc2292 (  0) 0) |
| [13] |
Takagi Y, Setoyama D, Ito R, et al. Human MTH3(NUDT18) protein hydrolyzes oxidized forms of guanosine and deoxyguanosine diphosphates:comparison with MTH1 and MTH2[J]. J Biol Chem, 2012, 287(25): 21541-21549. DOI:10.1074/jbc.M112.363010 (  0) 0) |
| [14] |
Kim HT, Choi R, Won HH, et al. NUDT15 genotype distributions in the Korean population[J]. Pharmacogenet Genomics, 2017, 27(5): 197-200. DOI:10.1097/FPC.0000000000000274 (  0) 0) |
| [15] |
Asada A, Nishida A, Shioya M, et al. NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease[J]. J Gastroenterol, 2016, 51(1): 22-29. DOI:10.1007/s00535-015-1142-4 (  0) 0) |
| [16] |
Hashiguchi K, Hayashi M, Sekiguchi M, et al. The roles of human MTH1, MTH2 and MTH3 proteins in maintaining genome stability under oxidative stress[J]. Mutat Res, 2018, 808: 10-19. DOI:10.1016/j.mrfmmm.2018.01.002 (  0) 0) |
| [17] |
Singh M, Bhatia P, Khera S. Emerging role of NUDT15 polymorphisms in 6-mercaptopurine metabolism and dose related toxicity in acute lymphoblastic leukaemia[J]. Leuk Res, 2017, 62: 17-22. DOI:10.1016/j.leukres.2017.09.012 (  0) 0) |
| [18] |
Moriyama T, Yang YL, Nishii R, et al. Novel variants inand thiopurine intolerance in children with acute lymphoblastic leukemia from diverse ancestry[J]. Blood, 2017, 130(10): 1209-1212. DOI:10.1182/blood-2017-05-782383 (  0) 0) |
| [19] |
Moriyama T, Nishii R, Perez-Andreu V, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity[J]. Nat Genet, 2016, 48(4): 367-373. DOI:10.1038/ng.3508 (  0) 0) |
| [20] |
Fong WY, Ho CC, Poon WT. Comparison of direct sequencing, real-time PCR-high resolution melt (PCR-HRM) and PCRrestriction fragment length polymorphism (PCR-RFLP) analysis for genotyping of common thiopurine intolerant variant alleles NUDT15 c415C>T and TPMT c719A>G (TPMT*3C)[J]. Diagnostics (Basel, Switzerland), 2017, 7(2): pii:E27. (  0) 0) |
| [21] |
Ho CC, Fong WY, Lee YH. Novel tetra-primer ARMS-PCR assays for thiopurine intolerance susceptibility mutations NUDT15 c415C>T and TPMT c719A>G (TPMT*3C) in East Asians[J]. Genes, 2017, 8(10): pii:E285. DOI:10.3390/genes8100285 (  0) 0) |
| [22] |
Yang JJ, Landier W, Yang W, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia[J]. J Clin Oncol, 2015, 33(11): 1235-1242. DOI:10.1200/JCO.2014.59.4671 (  0) 0) |
| [23] |
Zhang AL, Yang J, Wang H, et al. Association of NUDT15 c.415C>T allele and thiopurine-induced leukocytopenia in Asians:a systematic review and meta-analysis[J]. Ir J Med Sc, 2018, 187(1): 145-153. DOI:10.1007/s11845-017-1608-x (  0) 0) |
| [24] |
Sato T, Takagawa T, Kakuta Y, et al. NUDT15, FTO and RUNX1 genetic variants and thiopurine intolerance among Japanese patients with inflammatory bowel diseases[J]. Intest Res, 2017, 15(3): 328-337. DOI:10.5217/ir.2017.15.3.328 (  0) 0) |
| [25] |
Kim H, Seo H, Park Y, et al. APEX1 polymorphism and mercaptopurine-related early onset neutropenia in pediatric acute lymphoblastic leukemia[J]. Cancer Res Treat, 2018, 50(3): 823-834. DOI:10.4143/crt.2017.351 (  0) 0) |
| [26] |
Soler AM, Olano N, Méndez Y, et al. TPMT and NUDT15 genes are both related to mercaptopurine intolerance in acute lymphoblastic leukaemia patients from Uruguay[J]. Br J Haematol, 2018, 181(2): 252-255. DOI:10.1111/bjh.2018.181.issue-2 (  0) 0) |
| [27] |
Zgheib NK, Akika R, Mahfouz R, et al. NUDT15 and TPMT genetic polymorphisms are related to 6-mercaptopurine intolerance in children treated for acute lymphoblastic leukemia at the Children's Cancer Center of Lebanon[J]. Pediatr Blood Cancer, 2017, 64(1): 146-150. DOI:10.1002/pbc.v64.1 (  0) 0) |
| [28] |
Liang DC, Yang CP, Liu HC, et al. NUDT15 gene polymorphism related to mercaptopurine intolerance in Taiwan Chinese children with acute lymphoblastic leukemia[J]. Pharmacogenomics J, 2016, 16(6): 536-539. DOI:10.1038/tpj.2015.75 (  0) 0) |
| [29] |
Zhu X, Wang XD, Chao K, et al. NUDT15 polymorphisms are better than thiopurine S-methyltransferase as predictor of risk for thiopurine-induced leukopenia in Chinese patients with Crohn's disease[J]. Aliment Pharmacol Ther, 2016, 44(9): 967-975. DOI:10.1111/apt.2016.44.issue-9 (  0) 0) |
| [30] |
Brandalise SR, Pinheiro VR, Aguiar SS, et al. Benefits of the intermittent use of 6-mercaptopurine and methotrexate in maintenance treatment for low-risk acute lymphoblastic leukemia in children:randomized trial from the Brazilian Childhood Cooperative Group——protocol ALL-99[J]. J Clin Oncol, 2010, 28(11): 1911-1918. DOI:10.1200/JCO.2009.25.6115 (  0) 0) |
| [31] |
Asada A, Nishida A, Shioya M, et al. NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease[J]. J Gastroenterol, 2016, 51(1): 22-29. DOI:10.1007/s00535-015-1142-4 (  0) 0) |
| [32] |
Rudin S, Marable M, Huang RS, et al. The promise of pharmacogenomics in reducing toxicity during acute lymphoblastic leukemia maintenance treatment[J]. Genomics Proteomics Bioinformatics, 2017, 15(2): 82-93. DOI:10.1016/j.gpb.2016.11.003 (  0) 0) |
| [33] |
Moriyama T, Nishii R, Lin TN, et al. The effects of inherited NUDT15 polymorphisms on thiopurine active metabolites in Japanese children with acute lymphoblastic leukemia[J]. Pharmacogenet Genomics, 2017, 27(6): 236-239. DOI:10.1097/FPC.0000000000000282 (  0) 0) |
| [34] |
Lee YJ, Hwang EH, Park JH, et al. NUDT15 variant is the most common variant associated with thiopurine-induced early leukopenia and alopecia in Korean pediatric patients with Crohn's disease[J]. Eur J Gastroenterol Hepatol, 2016, 28(4): 475-478. (  0) 0) |
| [35] |
Tanaka Y, Kato M, Hasegawa D, et al. Susceptibility to 6-MP toxicity conferred by a NUDT15 variant in Japanese children with acute lymphoblastic leukaemia[J]. Br J Haematol, 2015, 171(1): 109-115. DOI:10.1111/bjh.2015.171.issue-1 (  0) 0) |
 2019, Vol. 21
2019, Vol. 21


