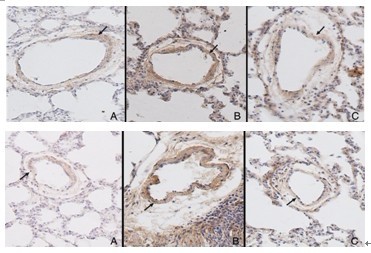
OBJECTIVE: To observe the effect of adrenomedullin (ADM) on the pulmonary vascular collagen metabolism in hypoxic rats in order to study the effect of ADM on chronic hypoxic pulmonary vascular structural remodeling and its possible mechanism. METHODS: Nineteen male Wistar rats were randomly divided into three groups: normal control (n=6), hypoxia (n=7) and ADM-treated hypoxia (n=6). ADM was subcutaneously administered into rats of the ADM-treated hypoxia group by mini-osmotic pump (300 ng/h) for two weeks. After two weeks of hypoxic challenge, mean pulmonary arterial pressure (mPAP) was evaluated using a right cardiac catheterization procedure. The ratio of right ventricular mass to left ventricular plus septal mass [RV/ (LV+S)] was measured. The changes of pulmonary vascular microstructure were observed. Meanwhile, the expression levels of collagen I, collagen III and transforming growth factor (TGF)-β in pulmonary arteries were detected by immunohistochemical assay. RESULTS: mPAP and RV/(LV+S) increased significantly in the hypoxia group compared with normal controls (P<0.01). The muscularization of small pulmonary vessels and the relative medial thickness of pulmonary arteries increased obviously in the hypoxia group compared with those in the normal control group (P<0.01). Meanwhile, the expression levels of collagen I, collagen III and TGF-β of pulmonary arteries in the hypoxia group increased markedly compared with those in the normal control group. However, mPAP and RV/(LV+S) were significantly reduced in the ADM-treated hypoxia group compared with those in the hypoxia group (P<0.01). ADM ameliorated pulmonary vascular structural remodeling of hypoxic rats, with a decrease in the expression of collagen I, collagen III and TGF-β of pulmonary arteries. CONCLUSIONS: ADM might play a regulatory role in the development of hypoxic pulmonary hypertension and hypoxic pulmonary vascular remodeling, through inhibiting the expression of TGF-β and alleviating the collagen accumulation of pulmonary arteries.
Key words
Adrenomedullin /
Pulmonary hypertension /
Hypoxia /
Collagen /
Rats
{{custom_sec.title}}
{{custom_sec.title}}
{{custom_sec.content}}
References
[1]杜军保, 唐朝枢. 肺动脉高压[M]. 北京:北京大学医学出版社, 2010:27-119.
[2]梁晨, 金红芳, 杜淑旭, 杜军保. 野百合碱诱导肺动脉高压大鼠肺动脉I型胶原的变化[J]. 实用儿科临床杂志,2010, 25(1):18-20.
[3]Ooi CY, Wang Z, Tabima DM, Eickhoff JC, Chesler NC. The role of collagen in extralobar pulmonary artery stiffening in response to hypoxia-induced pulmonary hypertension[J]. Am J Physiol Heart Circ Physiol, 2010, 299(6): H1823-H1831.
[4]Qi JG, Ding YG, Tang CS, Du JB. Chronic administration of adrenomedullin attenuates hypoxic pulmonary vascular structural remodeling and inhibits proadrenomedullin Nterminal 20-peptide production in rats[J]. Peptides, 2007, 28(4): 910-919.
[5]Murakami S, Kimura H, Kangawa K, Nagaya N. Physiological significance and therapeutic potential of adrenomedullin in pulmonary hypertension[J]. Cardiovasc Hematol Disord Drug Targets, 2006, 6(2): 125-132.
[6]Matsui H, Shimosawa T, Itakura K, Xing GQ, Ando K, Fujita T. Adrenomedullin can protect against pulmonary vascular remodeling induced by hypoxia[J]. Circulation, 2004, 109(18): 2246-2251.
[7]齐建光,李晓惠,丁亚光,唐朝枢,杜军保. 高肺血流性肺动脉高压大鼠肾上腺髓质素前体N端20肽的变化[J]. 中国当代儿科杂志,2007,9(6): 574-576.
[8]薛全福,谢剑鸣 胡长贵,邵茂刚,李长城. 常压缺氧性大鼠肺动脉高压模型的建立[J]. 中华结核和呼吸杂志,1989,12(6):350-351.
[9]田悦, 杜军保. 低氧性肺动脉高压发生机制中气体信号分子的病理生理学意义[J]. 中国当代儿科杂志, 2008, 10(1):98-101.
[10]Ishimitsu T, Ono H, Minami J, Matsuoka H. Pathophysiologic and therapeutic implications of adrenomedullin in cardiovascular disorders[J]. Phamacol Ther, 2006, 111(3): 909-927.
[11]Nakanishi K, Osada H, Uenoyama M, Kanazawa F, Ohrui N, Masaki Y, et al. Expression of adrenomedullin mRNA and protein in rats with hypobaric hypoxia-induced pulmonary hypertension[J]. Am J Physiol Heart Physiol, 2004, 286(6): H2159-H2168.
[12]Bobik A. Transforming growth factor-betas and vascular disorders[J]. Arterioscler Thromb Vasc Biol, 2006, 26(8): 1712-1720.
[13]Eickelberg O, K-hler E, Reichenberger F, Bertschin S, Woodtli T, Erne P, et al. Extracellular matrix deposition by primary human lung fibroblasts in response to TGF-beta1 and TGF-beta3[J]. Am J Physiol, 1999, 276 (5 Pt 1): L814-L824.
[14]Long L, Crosby A, Yang X, Southwood M, Upton PD, Kim DK, et al. Altered bone morphogenetic protein and transforming growth factor-beta signaling in rat models of pulmonary hypertension: potential for activin receptor-like kinase-5 inhibition in prevention and progression of disease[J]. Circulation, 2009, 119(4): 566-576.
 PDF(1302 KB)
PDF(1302 KB)


 PDF(1302 KB)
PDF(1302 KB)
 PDF(1302 KB)
PDF(1302 KB)