 PDF(2760 KB)
PDF(2760 KB)


Expression and significance of tight junction proteins in the kidney in a mouse model of renal ischemia-reperfusion injury
LI Zhi-Hui, XIA Tuan-Hong, KANG Zhi-Juan, DENG Xu, WANG Ying
Chinese Journal of Contemporary Pediatrics ›› 2018, Vol. 20 ›› Issue (12) : 1055-1062.
 PDF(2760 KB)
PDF(2760 KB)
 PDF(2760 KB)
PDF(2760 KB)
Expression and significance of tight junction proteins in the kidney in a mouse model of renal ischemia-reperfusion injury
Objective To study the expression and significance of tight junction proteins (claudin-2, claudin-10, and claudin-17) in a mouse model of renal ischemia-reperfusion injury.Methods A total of 152 male C57BL/6 mice were randomly assigned to control group (n=8), sham-operation group (n=72), and model group (n=72). The renal pedicles at both sides were clamped for 30 minutes to establish a mouse model of renal ischemia-reperfusion injury. According to the time points of reperfusion (0, 3, 6, 12, 24, 48, and 72 hours and 5 and 7 days), the sham-operation group and the model group were further divided into 9 subgroups, with 8 mice in each subgroup. RT-PCR and immunohistochemistry were used to measure the mRNA and protein expression of claudin-2, claudin-10, and claudin-17 in renal tissue.Results The control and sham-operation groups had no significant changes in the mRNA and protein expression of claudin-2, claudin-10, and claudin-17 in renal tissue over the time of reperfusion (P > 0.05). Compared with the control and sham-operation groups, the model group had decreased mRNA and protein expression of claudin-2 and claudin-10 after reperfusion, and the expression decreased gradually over the time of reperfusion, with the lowest levels at 24 hours of reperfusion (P < 0.05). Compared with the control and sham-operation groups, the model group had increased mRNA and protein expression of claudin-17 after reperfusion, and the expression increased gradually over the time of reperfusion, with the highest mRNA level at 12 hours and the highest protein level at 24 hours of reperfusion (P < 0.05).Conclusions Renal ischemia-reperfusion injury is closely associated with abnormal expression of tight junction proteins claudin-2, claudin-10, and claudin-17.
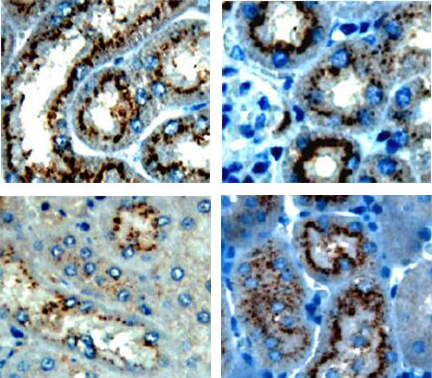
Acute kidney injury / Ischemia-reperfusion / Tight junction protein / Mice
[1] Mehta RL, Cerdá J, Burdmann EA, et al. International Society of Nephrology's 0 by 25 initiative for acute kidney injury (zero preventable deaths by 2025):a human rights case for nephrology[J]. Lancet, 2015, 385(9987):2616-2643.
[2] Selby NM, Fluck RJ, Kolhe NV, et al. International criteria for acute kidney injury:advantages and remaining challenges[J]. PLoS Med, 2016, 13(9):e1002122.
[3] Yang L, Xing G, Wang L, et al. Acute kidney injury in China:a cross-sectional survey[J]. Lancet, 2015, 386(10002):1465-1471.
[4] Pressly JD, Park F. DNA repair in ischemic acute kidney injury[J]. Am J Physiol Renal Physiol, 2017, 312(4):F551-F555.
[5] Zuk A, Bonventre JV. Acute kidney injury[J]. Annu Rev Med, 2016, 67:293-307.
[6] Eadon MT, Hack BK, Xu C, et al. Endotoxemia alters tight junction gene and protein expression in the kidney[J]. Am J Physiol Renal Physiol, 2012, 303(6):F821-F830.
[7] Hou J. The kidney tight junction[J]. Int J Mol Med, 2014, 34(6):1451-1457.
[8] Krug SM, Schulzke JD, Fromm M. Tight junction, selective permeability, and related diseases[J]. Semin Cell Dev Biol, 2014, 36:166-176.
[9] Krug SM, Günzel D, Conrad MP, et al. Claudin-17 forms tight junction channels with distinct anion selectivity[J]. Cell Mol Life Sci, 2012, 69(16):2765-2778.
[10] Reyes JL, Molina-Jijón E, Rodríguez-Muñoz R, et al. Tight junction proteins and oxidative stress in heavy metals-induced nephrotoxicity[J]. Biomed Res Int, 2013, 2013:730-789.
[11] Trujillo J, Molina-Jijón E, Medina-Campos ON, et al. Renal tight junction proteins are decreased in cisplatin-induced nephrotoxicity in rats[J]. Toxicol Mech Methods, 2014, 24(7):520-528.
[12] 张良, 李志辉, 邓旭, 等. 紧密连接蛋白claudin-2在急性肾损伤患儿肾组织中的表达及意义[J]. 中国当代儿科杂志, 2014, 16(4):361-365.
[13] Ohta H, Chiba S, Ebina M, et al. Altered expression of tight junction molecules in alveolar septa in lung injury and fibrosis[J]. Am J Physiol Lung Cell Mol Physiol, 2012, 302(2):L193-L205.
[14] Laukoetter MG, Nava P, Lee WY, et al. JAM-A regulates permeability and inflammation in the intestine in vivo[J]. J Exp Med, 2007, 204(13):3067-3076.
[15] Krug SM, Günzel D, Conrad MP, et al. Claudin-17 forms tight junction channels with distinct anion selectivity[J]. Cell Mol Life Sci, 2012, 69(16):2765-2778.
[16] Li Z, Deng X, Kang Z, et al. Elevation of miR-21, through targeting MKK3, may be involved in ischemia pretreatment protection from ischemia-reperfusion induced kidney injury[J]. J Nephrol, 2016, 29(1):27-36.
[17] Yamaguti PM, Dos Santos PA, Leal BS, et al. Identification of the first large deletion in the CLDN16 gene in a patient with FHHNC and late-onset of chronic kidney disease:case report[J]. BMC Nephrol, 2015, 16(1):92.
[18] Krug SM, Schulzke JD, Fromm M. Tight junction, selective permeability, and related diseases[J]. Semin Cell Dev Biol, 2014, 36:166-176.
[19] Gong Y, Yu M, Yang J, et al. The Cap1-claudin-4 regulatory pathway is important for renal chloride reabsorption and blood pressure regulation[J]. Proc Natl Acad Sci U S A, 2014, 111(36):E3766-E3774.
[20] Schnermann J, Huang Y, Mizel D. Fluid reabsorption in proximal convoluted tubules of mice with gene deletions of claudin-2 and/or aquaporin1[J]. Am J Physiol Renal Physiol, 2013, 305(9):1352-1364.
[21] Kieran NE, Doran PP, Connolly SB, et al. Modification of the transcriptomic response to renal ischemia/reperfusion injury by lipoxin analog[J]. Kidney Int, 2003, 64(2):480-492.
[22] Gao M, Li W, Wang H, et al. The distinct expression patterns of claudin-10, -14, -17 and E-cadherin between adjacent non-neoplastic tissues and gastric cancer tissues[J]. Diagn Pathol, 2013, 8:205.