 PDF(1078 KB)
PDF(1078 KB)


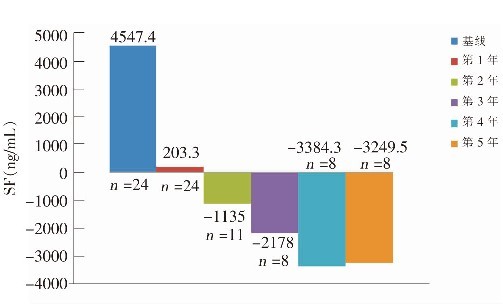
Curative effects and safety of deferasirox in treatment of iron overload in children with β-thalassemia major
GAO Hong-Ying, LI Qi, CHEN Juan-Juan, CHEN Guang-Fu, LI Chang-Gang
Chinese Journal of Contemporary Pediatrics ›› 2011, Vol. 13 ›› Issue (7) : 531-534.
 PDF(1078 KB)
PDF(1078 KB)
 PDF(1078 KB)
PDF(1078 KB)
Curative effects and safety of deferasirox in treatment of iron overload in children with β-thalassemia major
β-thalassemia major / Deferasirox / Serum feritin / Iron overload / Child