 PDF(1188 KB)
PDF(1188 KB)


Nasopharyngeal carriage rate, antimicrobial resistance and serotype distribution of Streptococcus pneumoniae among children with upper respiratory infection
YU Sang-Jie, GAO Wei, SHI Wei, YUAN Lin, SHEN A-Dong, YAO Kai-Hu, YANG Yong-Hong
Chinese Journal of Contemporary Pediatrics ›› 2014, Vol. 16 ›› Issue (10) : 988-992.
 PDF(1188 KB)
PDF(1188 KB)
 PDF(1188 KB)
PDF(1188 KB)
Nasopharyngeal carriage rate, antimicrobial resistance and serotype distribution of Streptococcus pneumoniae among children with upper respiratory infection
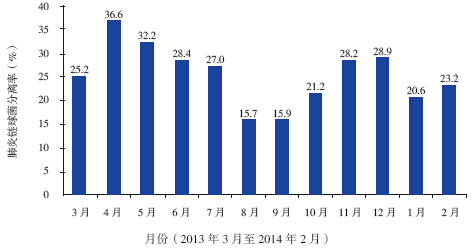
Objective To investigate nasopharyngeal carriage rate, antimicrobial resistance and serotype distribution of Streptococcus pneumoniae among children with upper respiratory infection. Methods Nasopharygeal swabs were collected from children with upper respiratory infection visiting the outpatient department of Beijing Children's Hospital between March 2013 and February 2014. The antibiotic susceptibility was tested by Etest method, and the serotype was determined by Quellung reaction. Results The nasopharyngeal carriage rate for Streptococcus pneumoniae was 23.8% (699/2 941). One hundred isolates were randomly chosen for antimicrobial susceptiblity test and serotyping. Up to 98.0% isolates were susceptible to parenteral penicillin. The susceptible rate against oral penicillin, however, was 33.0%. The non-susceptible rate to erythromycin and azithromycin was 97.0%. The multi-drug resistance rate was up to 86.0%. The common serotypes were 6A(12.0%), 19F(12.0%), 6B(10.0%), 23F(9.0%) and 14(8.0%). The coverage rates of 7-, 10- and 13-valent pneumococcal conjugate vaccine were 41.0%, 42.0% and 59.0% respectively. Conclusions About 25% of children with upper respiratory infection are nasopharyngeal colonized by Streptococcus pneumoniae. The isolates show a high antimicrobial resistance. The 13-valent pneumococcal conjugate vaccine covers about 60.0% of the isolates.
Streptococcus pneumoniae / Carriage rate / Antimicrobial resistance / Serotype / Child