 PDF(2939 KB)
PDF(2939 KB)


Establishment of cardiac remodeling model in FVB/N mice by intraperitoneal injection of isoproterenol
YUAN Yong-Hua, ZHENG Xue-Ming, HE Xue-Hua, LIU Li-Ping, XU Wei, XIA Xiao-Hui, LUO Jian-Hong, LYU Mei, ZHU Qian-Li, WANG Sheng, WU Shi
Chinese Journal of Contemporary Pediatrics ›› 2018, Vol. 20 ›› Issue (6) : 508-513.
 PDF(2939 KB)
PDF(2939 KB)
 PDF(2939 KB)
PDF(2939 KB)
Establishment of cardiac remodeling model in FVB/N mice by intraperitoneal injection of isoproterenol
Objective To explore the feasibility of intraperitoneal injection of isoproterenol (ISO) to induce cardiac remodeling in FVB/N mice. Methods Forty-eight FVB/N mice were divided into back subcutaneous saline group (subcutaneous saline group), intraperitoneal saline group, back subcutaneous ISO group (subcutaneous ISO group), and intraperitoneal ISO group according to the route of administration of saline or ISO. ISO (30 μg/g body weight/day) was given to the subcutaneous ISO group and the intraperitoneal ISO group, twice daily with an interval of 12 hours, for 14 consecutive days. The subcutaneous saline group and the intraperitoneal saline group were injected with an equal volume of saline. The left ventricular end-diastolic posterior wall thickness was measured by echocardiography, and the ratio of heart weight to tibia length was determined. Hematoxylin-eosin staining was used to determine the myocardial fiber diameter. Picric-sirius red staining was used to determine the myocardial collagen deposition area. Quantitative real-time PCR was used to measure the mRNA expression of collagen I. Results Compared with the subcutaneous ISO, subcutaneous saline, and intraperitoneal saline groups, the intraperitoneal ISO group had increased sizes of the cardiac cavity and the heart. Compared with the subcutaneous saline and intraperitoneal saline groups, the subcutaneous ISO group showed no significant changes in the gross morphology of the cardiac cavity and the heart. The intraperitoneal ISO group showed significant increases in the ratio of heart weight to tibia length, myocardial fiber diameter, left ventricular end-diastolic posterior wall thickness, myocardial collagen area percentage, and the mRNA expression of collagen I compared with the subcutaneous ISO, subcutaneous saline, and intraperitoneal saline groups (P < 0.01). There were no significant differences in the above five indices between the subcutaneous ISO group and the subcutaneous saline and intraperitoneal saline groups (P > 0.05). No significant difference in the mortality rate was found between the subcutaneous ISO and intraperitoneal ISO groups (P > 0.05). Conclusions Intraperitoneal injection of ISO can induce cardiac hypertrophy and fibrosis in FVB/N mice.
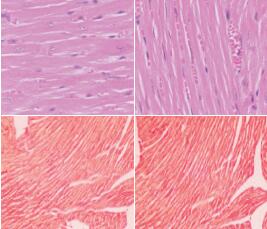
Cardiac remodeling / Cardiac hypertrophy / Fibrosis / Isoproterenol / Mice
[1] Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2015 update:a report from the American Heart Association[J]. Circulation, 2015, 131(4):e29-e322.
[2] 王文, 朱曼璐, 王拥军, 等. 《中国心血管病报告2012》概要[J]. 中国循环杂志, 2013, 28(6):408-412.
[3] Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis[J]. Cell Mol Life Sci, 2014, 71(4):549-574.
[4] Li C, Huang D, Tang J, et al. ClC-3 chloride channel is involved in isoprenaline-induced cardiac hypertrophy[J]. Gene, 2018, 642:335-342.
[5] Krum H, Roecker EB, Mohacsi P, et al. Effects of initiating carvedilol in patients with severe chronic heart failure:results from the COPERNICUS Study[J]. JAMA, 2003, 289(6):712-718.
[6] Taketo M, Schroeder AC, Mobraaten LE, et al. FVB/N:an inbred mouse strain preferable for transgenic analyses[J]. Proc Natl Acad Sci U S A, 1991, 88(6):2065-2069.
[7] 杨承志, 田爱炬, 孟增慧, 等. 异丙肾上腺素诱导FVB/N小鼠心脏肥大模型的建立[J]. 北京大学学报(医学版), 2014, 46(6):906-910.
[8] 袁勇华, 何学华, 方亦兵, 等. 黄芪对扩张型心肌病大鼠心肌缝隙连接蛋白43表达的影响[J]. 临床儿科杂志, 2014, 32(11):1080-1083.
[9] Chaulet H, Lin F, Guo J, et al. Sustained augmentation of cardiac alpha1A-adrenergic drive results in pathological remodeling with contractile dysfunction, progressive fibrosis and reactivation of matricellular protein genes[J]. J Mol Cell Cardiol, 2006, 40(4):540-552.
[10] Fu Y, Xiao H, Zhang Y. Beta-adrenoceptor signaling pathways mediate cardiac pathological remodeling[J]. Front Biosci (Elite Ed), 2012, 4:1625-1637.
[11] Aflaki M, Qi XY, Xiao L, et al. Exchange protein directly activated by cAMP mediates slow delayed-rectifier current remodeling by sustained β-adrenergic activation in guinea pig hearts[J]. Circ Res, 2014, 114(6):993-1003.
[12] Chowdhury D, Tangutur AD, Khatua TN, et al. A proteomic view of isoproterenol induced cardiac hypertrophy:prohibitin identified as a potential biomarker in rats[J]. J Transl Med, 2013, 11:130.
[13] Lv T, Du Y, Cao N, et al. Proliferation in cardiac fibroblasts induced by β1-adrenoceptor autoantibody and the underlying mechanisms[J]. Sci Rep, 2016, 6:32430.
[14] van Berlo JH, Maillet M, Molkentin JD. Signaling effectors underlying pathologic growth and remodeling of the heart[J]. J Clin Invest, 2013, 123(1):37-45.
[15] Cramariuc D, Gerdts E. Epidemiology of left ventricular hypertrophy in hypertension:implications for the clinic[J]. Expert Rev Cardiovasc Ther, 2016, 14(8):915-926.
[16] Turillazzi E, Neri M, Riezzo I, et al. Cardiac fibrosis, arrhythmia and sudden death in myotonic dystrophy type 1:could TGF-ß1 improve the predictive accuracy of patients at risk, opening new therapeutic challenges?[J]. Int J Cardiol, 2013, 168(5):4976-4978.
[17] Grimaldi V, De Pascale MR, Zullo A, et al. Evidence of epigenetic tags in cardiac fibrosis[J]. J Cardiol, 2017, 69(2):401-408.
[18] Okin PM, Hille DA, Kjeldsen SE, et al. Persistence of left ventricular hypertrophy is associated with increased cardiovascular morbidity and mortality in hypertensive patients with lower achieved systolic pressure during antihypertensive treatment[J]. Blood Press, 2014, 23(2):71-80.
[19] Wang LS, Lee CT, Su WL, et al. Delonix regia leaf extract (DRLE):a potential therapeutic agent for cardioprotection[J]. PLoS One, 2016, 11(12):e0167768.
[20] Iaccarino G, Tomhave ED, Lefkowitz RJ, et al. Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by beta-adrenergic receptor stimulation and blockade[J]. Circulation, 1998, 98(17):1783-1789.
[21] Duan Q, Ni L, Wang P, et al. Deregulation of XBP1 expression contributes to myocardial vascular endothelial growth factor-A expression and angiogenesis during cardiac hypertrophy in vivo[J]. Aging Cell, 2016, 15(4):625-633.
[22] Song S, Si LY. Klotho ameliorated isoproterenol-induced pathological changes in cardiomyocytes via the regulation of oxidative stress[J]. Life Sci, 2015, 135:118-123.
[23] Huang ZP, Chen J, Seok HY, et al. MicroRNA-22 regulates cardiac hypertrophy and remodeling in response to stress[J]. Circ Res, 2013, 112(9):1234-1243.
[24] 姜国良, 于晓, 徐恺, 等. 腹腔和皮下注射D-半乳糖衰老大鼠模型分析[J]. 中国老年学杂志, 2013, 33(5):1101-1103.
[25] 龚频, 陈福欣, 金赛, 等. 不同给药方式下蓝莓花青素的镇痛作用研究[J]. 食品研究与开发, 2013, 34(24):186-188.
[26] 徐南平, 汪倩, 邹音, 等. 表皮生长因子对多器官功能障碍小鼠的作用[J]. 中华急诊医学杂志, 2012, 21(5):497-502.
[27] 邹磊, 李一鸣, 杨建云, 等. 甾体类固醇衍生物AGE对60Coγ射线辐射C57小鼠治疗作用[J]. 科学技术与工程, 2015, 15(17):29-33.
[28] 秦文艳, 陈贺, 朱竟赫, 等. 不同氢化可的松小鼠肾阳虚模型制备方法对比研究[J]. 实验动物科学, 2017, 34(4):11-14.
[29] 安祯祥, 何远利, 王敏. 四氯化碳不同给药途径复合乙醇诱导大鼠肝纤维化模型的研究[J]. 贵州医药, 2016, 40(1):6-8.