 PDF(1288 KB)
PDF(1288 KB)


急性和慢性肺损伤的模式及病因:基于非临床性实验证据的剖析
Matthias C. Hütten, Boris W. Kramer
中国当代儿科杂志 ›› 2014, Vol. 16 ›› Issue (5) : 448-459.
 PDF(1288 KB)
PDF(1288 KB)
 PDF(1288 KB)
PDF(1288 KB)
急性和慢性肺损伤的模式及病因:基于非临床性实验证据的剖析
Patterns and etiology of acute and chronic lung injury:insights from experimental evidence
Adequate pulmonary function is pivotal for preterm infants. Besides being structurally immature, the preterm lung is susceptible to injury resulting from different prenatal conditions and postnatal insults. Lung injury might result in impaired postnatal lung development, contributing to chronic lung disease of prematurity, bronchopulmonary dysplasia (BPD). This review focuses on lung injury mediated by and related to inflammatory changes in the lung. We give an overview on experimental models which have helped to elucidate mechanisms of pulmonary inflammation in prematurity. We describe experimental data linking acute and chronic chorioamnionitis with intrapulmonary inflammation, lung maturation and surfactant production in various animal models. In addition, experimental data has shown that fetal inflammatory response is modulated by the fetus himself. Experimental data has therefore helped to understand differential effects on lung function and lung maturation exerted by maternal administration of potentially anti-inflammatory substances like glucocorticosteroids (GCS). New approaches of modulation of pulmonary inflammation/injury caused by postnatal interventions during resuscitation and mechanical ventilation have been studied in animal models. Postnatal therapeutic interventions with widely used drugs like oxygen, steroids, surfactant, caffeine and vitamin A have been experimentally and mechanistically assessed regarding their effect on pulmonary inflammation and lung injury. Carefully designed experiments will help to elucidate the complex interaction between lung injury, lung inflammation, repair and altered lung development, and will help to establish a link between lung alterations originating in this early period of life and long-term adverse respiratory effects.
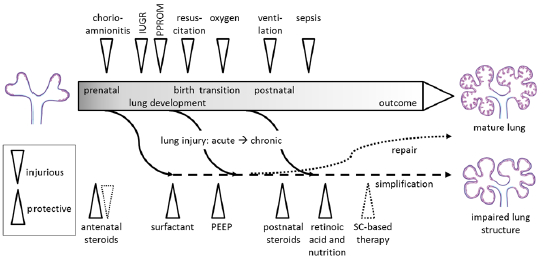
Lung injury / Etiology / Pulmonary inflammation / Chorioamnionitis / Preterm infant
Lung injury / Etiology / Pulmonary inflammation / Chorioamnionitis / Preterm infant