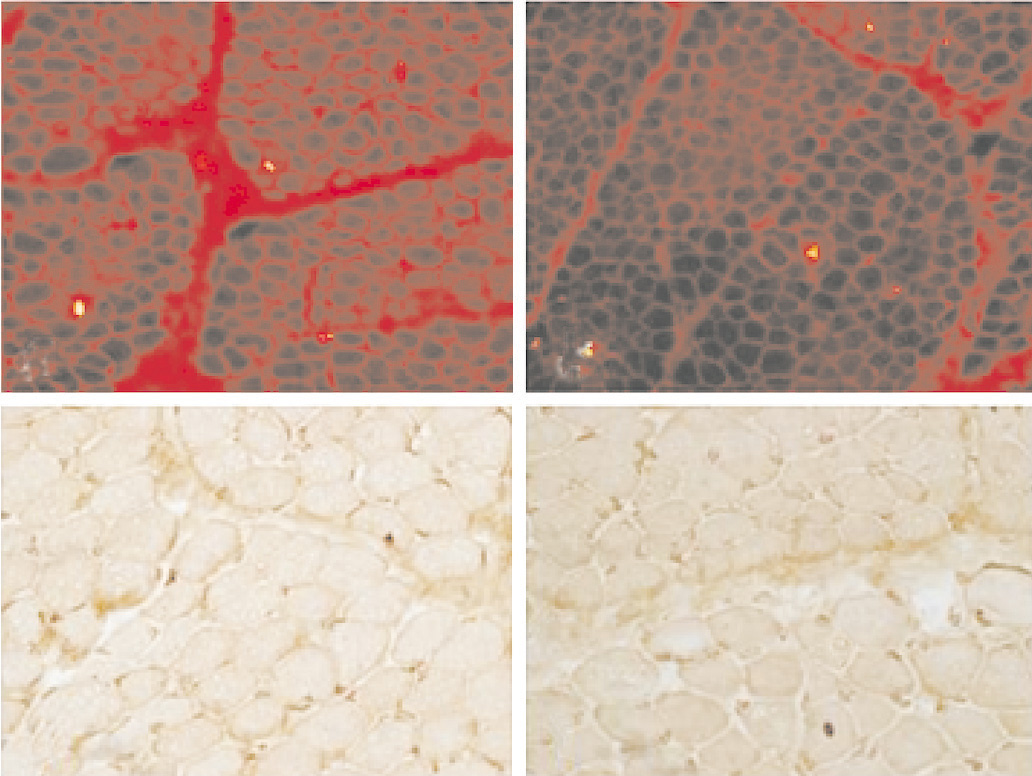
目的:利用免疫组织化学技术检测肌细胞膜上Dystrophin蛋白表达是鉴别诊断Duchenne型和 Becker型肌营养不良(DMD和BMD)的病理学基础,然而个别病例的肌细胞膜上Dystrophin蛋白表达几乎接近于正常,这给临床疑似BMD诊断带来一定困难。本研究探讨神经源性一氧化氮合酶(nNOS)对BMD患者的诊断价值。方法:对 Dys-C 端结构域表达呈阳性的 5 例BMD(其中已临床明确诊断 3 例,临床疑似 2 例)和作为对照的骨折患儿肌肉组织,利用免疫组织化学的方法行单克隆 Dytrophin 抗体和 nNOS 抗体染色。结果:与对照组比较,已临床明确诊断BMD 3例病例的肌细胞膜上 Dys-C、Dys-R 和 Dys-N 端结构域蛋白均有显著缺乏,nNOS 均未见表达;临床疑似 2 例 BMD 病例的肌细胞膜上,即使 Dystrophin蛋白接近于正常表达,但 nNOS 在肌细胞膜上是缺乏的。结论:对 Dystrophinn 三端结构域蛋白接近于正常表达的临床疑似BMD患儿,可通过nNOS抗体染色达到明确诊断的目的。
Abstract
OBJECTIVE: Immunohistochemistry using antibodies to dystrophin is the pathological basis for the differential diagnosis of Duchenne and Becker muscular dystrophy (DMD and BMD). In rare cases, however, labelling dystrophin on sarcolemma is equivocal and similar to that observed in controls. This makes the diagnosis of BMD difficult. This study aimed to explore the diagnostic value of neuronal nitric oxide synthase (nNOS) antibody for clinically suspected BMD. METHODS: Immunohistochemical staining was performed on muscle specimens of 5 cases of BMD with positive expression of Dys-C (3 cases had a confirmed diagnosis of BMD, 2 cases were clinically suspected as BMD) by using dystrophin and nNOS antibodies. Normal muscle specimens from the children with fracture were used as controls. RESULTS: Compared with the controls, the expression of Dys-R, Dys-C and Dys-N was markedly reduced and nNOS was not expressed on sarcolemma in the three cases of definitely diagnosed BMD. The two cases of clinically suspected as BMD had a complete absence of sarcolemmal nNOS, even if had a similar expression of dystrophin on sarcolemma to the controls. CONCLUSIONS: nNOS antibody staining can be used for a definite diagnosis in children with clinically suspected BMD who have the almost normal expression of dystrophin.
关键词
神经源性一氧化氮合酶 /
抗肌萎缩蛋白 /
Becker型肌营养不良 /
儿童
Key words
Neuronal nitric oxide synthase /
Dystrophin /
Becker muscular dystrophy /
Child
{{custom_sec.title}}
{{custom_sec.title}}
{{custom_sec.content}}
参考文献
[1]Dubowitz V, Sewry CA. Muscle biopsy: a practical approach[M]. 3rd ed. Philadelphia: Elsevier, 2006: 157-180.
[2]Deburgrave N, Daoud F, Liense S, Barbot JC, Récan D, Peccate C, et al. Protein-and mRNA-based phenotype-genotype correlations in DMD/BMD with point mutations and molecular basis for BMD with nonsense and frameshift mutations in the DMD gene[J]. Hum Mutat, 2007, 28(2): 183-195.
[3]Gualandi F, Neri M, Bovolenta M, Martoni E, Rimessi P, Fini S, et al. Transcriptional behavior of DMD gene duplications in DMD/BMD males[J]. Hum Mutat, 2009, 30(2): E310-E319.
[4]Torelli S, Brown SC, Jimenez-Mallebrera C, Feng L, Muntoni F, Sewry CA. Absence of neuronal nitric oxide synthase (nNOS) as a pathological marker for the diagnosis of Becker muscular dystrophy with rod domain deletions[J]. Neuropathol Appl Neurobiol, 2004, 30(5): 540-545.
[5]Arechavala Gomeza V, Kinali M, Feng L, Brown SC, Sewry C, Morgan JE, et al. Immunohistological intensity measurements as a tool to assess sarcolemma-associated protein expression[J]. Neuropathol Appl Neurobiol, 2010, 36(4): 265-274.
[6]李西华,徐苓,刘晓青,赵蕾,吴燕,沈莹,等. 对不同年龄阶段DMD患儿肌细胞膜上DGC关联复合物蛋白的分析[J]. 临床儿科杂志,2009,11(26):1040-1044.
[7]Lai Y, Thomas GD, Yue Y, Yang HT, Li D, Long C, et al. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy[J]. J Clin Invest, 2009, 119(3):624-635.
[8]Ségalat L, Grisoni K, Archer J, Vargas C, Bertrand A, Anderson JE. CAPON expression in skeletal muscle is regulated by position, repair, NOS activity, and dystrophy[J]. Exp Cell Res, 2005, 302(2): 170-179.
 PDF(1485 KB)
PDF(1485 KB)


 PDF(1485 KB)
PDF(1485 KB)
 PDF(1485 KB)
PDF(1485 KB)