 PDF(1411 KB)
PDF(1411 KB)


短发夹RNA沉默Tim3分子表达对哮喘小鼠外周血Th1和Th17细胞的影响
陆小霞,徐佳莉,董宗祈,陈鹏,王莹
中国当代儿科杂志 ›› 2013, Vol. 15 ›› Issue (4) : 302-307.
 PDF(1411 KB)
PDF(1411 KB)
 PDF(1411 KB)
PDF(1411 KB)
短发夹RNA沉默Tim3分子表达对哮喘小鼠外周血Th1和Th17细胞的影响
Small hairpin RNA silencing Tim-3 affects peripheral blood Th1 and Th17 cells differentiation in asthmatic mice
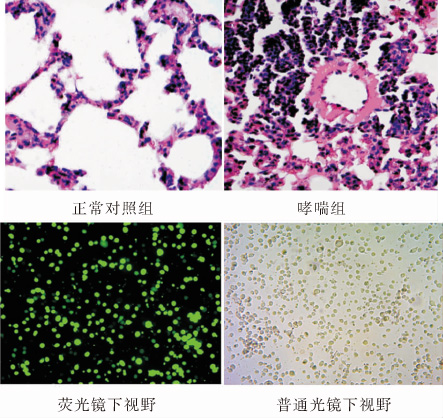
目的:建立小鼠支气管哮喘模型,采用T 细胞免疫球蛋白黏蛋白分子-3(Tim-3)特异短发夹RNA(shRNA)沉默外周血单个核细胞(PBMCs)中Tim-3的表达,探讨Tim-3对辅助性T细胞1 (Th1)和Th17细胞分化的影响。方法:用卵白蛋白(OVA)致敏并激发建立哮喘小鼠模型,分离哮喘小鼠PBMCs,用 Tim-3特异的shRNA 片段沉默哮喘小鼠PBMCs中高表达的Tim-3 基因。Real-time PCR 和 Western blot 检测 Tim-3 的表达,流式细胞分析技术(FACS)检测Th1和Th17的比例;ELISA 检测细胞培养上清液中干扰素γ(IFN-γ),白介素-4(IL-4),和 白介素-17(IL-17)的水平。结果:哮喘组小鼠PBMCs中Tim-3 mRNA表达明显升高; 使用特异Tim-3 shRNA沉默哮喘小鼠PBMCs后,沉默组PBMCs中Th1细胞比例显著升高,Th17细胞比例显著下降,与阴性对照组相比差异有统计学意义(P<0.01);沉默组PBMCs培养上清液中IFN-γ明显升高,IL-17明显降低,与阴性对照组相比差异有统计学意义(P<0.05), IL- 4水平无明显变化。结论:特异Tim-3 shRNA有效地沉默了Tim-3的表达,Tim-3表达的改变影响了T 细胞的分化。
OBJECTIVE: To investigate the effects of down-regulating Tim-3 gene in the peripheral blood mononuclear cells (PBMCs) of an asthmatic mouse model by short hairpin RNA (shRNA) and to explore the effect of Tim-3 on Th1 and Th17 cell differentiation. METHODS: An asthmatic murine model was established by ovalbumin sensitization and challenge. PBMCs were isolated from asthmatic mice and transfected by shRNA targeting Tim-3 gene. The mRNA and protein expressions of Tim-3 were detected by quantitative PCR and Western blot. Flow cytometry analysis was performed to determine the levels of Th1 and Th17, and ELISA was performed to determine concentrations of IFN-γ, IL-4 and IL-17 in the supernatant. RESULTS: Tim-3 mRNA expression in PBMCs was significantly increased in asthmatic mice. The mRNA and protein expression of Tim-3 decreased significantly in the shRNA group. Compared with the negative groups, Th1 cell levels increased and Th17 cell levels decreased significantly in the asthmatic groups after Tim-3 shRNA interference. In the Tim-3 shRNA interference groups concentrations of IFN-γ increased significantly while IL-17 decreased significantly. CONCLUSIONS: Specific Tim-3 shRNA effectively silences the expression of Tim-3 and change in Tim-3 expression could affect T cell differentiation.
Asthma / Tim-3 / RNA interference / T helper cells / Mice
[1]Bryan SA, O′Connor BJ, Matti S, Leckie MJ, Kanabar V, Khan J, et al. Effects of recombinant human interleukin-12 on eosinophils, airway hyper-responsiveness, and the late asthmatic response[J]. Lancet, 2000, 356(9248): 2149-2153.
[2]Cho SH, Stanciu LA, Holgate ST, Johnston SL. Increased interleukin-4, interleukin-5, and interferon-gamma in airway CD4+ and CD8+ T cells in atopic asthma[J]. Am J Respir Crit Care Med, 2005, 171(3): 224-230.
[3]Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages[J]. Nat Immunol, 2005, 6 (11): 1123-1132.
[4]Hastings WD, Anderson DE, Kassam N, Koguchi K, Greenfield EA, Kent SC, et al. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines[J]. Eur J Immunol, 2009, 39 (9): 2492-2501.
[5]Kearley J, McMillan SJ, Lioyd CM. Th2-driven, allergen-induced airway inflammation is reduced after treatment in vivo[J]. J Exp Med, 2007, 204 (6): 1289-1294.
[6]North ML, Khanna N, Marsden PA, Grasemann H, Scott JA. Functionally important role for arginase 1 in the airway hyperresponsiveness of asthma[J]. Am J Physiol Lung Cell Mol Physiol, 2009, 296(6): L911-L920.
[7]Zhao Y, Yang J, Gao YD, Guo W.Th17 immunity in patients with allergic asthma[J].Int Arch Allergy Immunol, 2010, 151(4): 297-307.
[8]Agache I, Ciobanu C, Agache C, Anghel M. Increased serum IL-17 is an independent risk factor for severe asthma[J]. Respir Med, 2010, 104(8): 1131-1137.
[9]Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness[J]. Am J Respir Crit Care Med, 2009, 180(8): 720-730.
[10]Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I,et al. Antigen-Specific T Cell Sensitization Is Impaired in IL-17-Deficient Mice, Causing Suppression of Allergic Cellular and Humoral Responses[J]. Immunity, 2002, 17(3): 375-387.
[11]胡斯明,罗雅玲,赖文岩.地塞米松对哮喘小鼠肺组织中转录因子RORγt mRNA表达的干预作用[J]. 细胞与分子免疫学杂志,2009, 25(12): 1115-1118.
[12]Nakae S, Iwakura Y, Suto H, Galli SJ. Phenotypic differences between Th1 and Th17 cells and negative regulation of Th1 cell differentiation by IL-17[J]. J Leukocyte Biol, 2007, 81(5): 1258-1268.
[13]陈伟超,刘恩梅,邓昱,何云,陈杰华,刘玮,等.新生期卡介苗接种对实验性哮喘小鼠肺 Th17细胞和IL17的影响[J].中国当代儿科杂志,2010,12(8):650-653.
[14]Kurschus FC, Croxford AL, Heinen AP, Wortge S, Lelo D, Waisman A. Genetic Proof for the transient nature of the Th17 phenotype[J]. Eur J Immunol, 2010, 40(12): 3336-3346.
[15]Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease[J]. Nature, 2002, 415 (6871): 536-541.
[16]张志英,栾斌,冯晓霞.Galectin-9及其受体 Tim-3在哮喘小鼠肺组织中的表达[J].中国当代儿科杂志,2011,13(5):406-410.
[17]Darcan-Nicolaisen Y,Meinicke H,Fels G, Hegend O, Haberland A, Kuhl A, et al. Small interfering RNA against transcription factor STAT6 inhibits allergic airway inflammation and hyperreactivity in mice[J]. Immunol, 2009, 182(12): 7501-7508.
[18]Moriwaki A, Inoue H, Nakano T, Matsunaga Y, Matsuno Y, Matsumoto T, et al. T Cell treatment with small-interfering RNA for suppressor of cytokine signaling 3 modulates allergic airway responses in a Murine Model of Asthma[J]. Am J Respir Cell Mol Biol, 2011, 44(4): 448-455.
[19]Bernards R, Brummelkamp TR, Beijersbergen RL. shRNA libraries and their use in cancer genetics[J]. Nat Methods, 2006, 3(9):701-706.