 PDF(1560 KB)
PDF(1560 KB)


 PDF(1560 KB)
PDF(1560 KB)
 PDF(1560 KB)
PDF(1560 KB)
脊髓内MAPK-ERK通路在心肌缺血再灌注损伤中的作用
Role of spinal MAPK-ERK signal pathway in myocardial ischemia-reperfusion injury
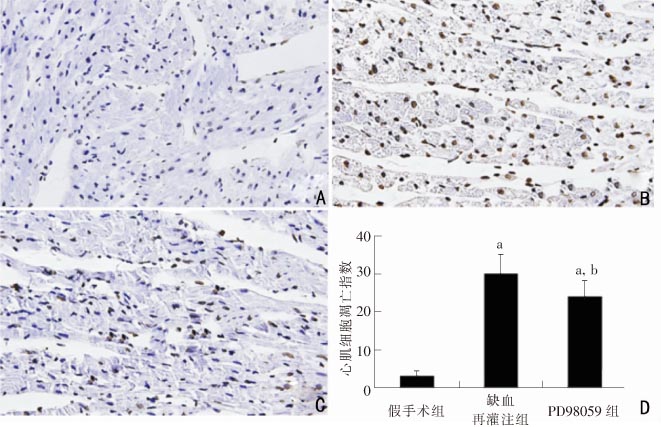
目的:探讨脊髓内MAPK-ERK通路在幼年大鼠心肌缺血再灌注损伤中的作用。方法:将鞘内置管成功的60只Sprague-Dawley(SD)幼年雄性大鼠(80~100 g)随机分为3组:假手术组(n=10)、缺血再灌注组(n=25)和PD98059组(n=25)。假手术组实验前30 min鞘内注射5 μL DMSO,开胸后冠状动脉左前降支备线但不结扎;缺血再灌注组于实验前30 min鞘内注射5 μL DMSO,开胸后结扎冠状动脉左前降支上1/3段30 min,再灌注120 min;PD98059组于实验前30 min鞘内注射5 μL PD98059(5 μg),余处理同缺血再灌注组。实验结束后取3组大鼠T1~T4段脊髓,免疫荧光法检测p-ERK表达情况。取3组大鼠心脏,分别检测其心肌梗死面积和缺血交界区心肌凋亡指数。结果:缺血再灌注组大鼠脊髓内p-ERK表达水平显著高于假手术组和PD98059组(P<0.05);PD98059组心肌凋亡指数和心肌梗死面积均明显低于缺血再灌注组(P<0.05),但仍高于假手术组(P<0.05)。结论:上胸段脊髓内MAPK-ERK通路在心肌缺血再灌注损伤中激活,阻断该通路可以产生明显的心肌保护作用。
OBJECTIVE: To explore the role of spinal MAPK-ERK signal pathway in myocardial ischemia-reperfusion (I/R) injury. METHODS: Sixty male Sprague-Dawley(SD) rats (80-100 g) were randomly divided into 3 groups: sham (n=10), PD98059 (n=25) and I/R groups (n=25). Three days after successful intrathecal implantation, 5 μg DMSO was injected intrathecally into the sham group, and then the left coronary arteries were separated without being tied. Rats in the I/R and PD98059 groups were injected with 5 μL DMSO and PD98059 (5 μg) 30 minutes before thoractomy respectively. Then the left coronary artery was tied for 30 minutes followed by 120 minutes of reperfusion. After the experiments, the ERK phosphorylation condition of T1-T4 spinal cord segments was detected with immunofluorescence; the myocardiac apoptosic index and infarct size were measured. RESULTS: Expression of p-ERK in the I/R group was significantly higher than in the sham and PD98059 groups (P<0.05). Myocardial apoptotic index and infarct size in the PD98059 group were significantly lower than in the I/R group (P<0.05), but higher in the PD98059 group than in the sham group (P<0.05). CONCLUSIONS: The MAPK-ERK pathway in the superior thorathic spinal cord can be activated by myocardial ischemia-reperfusion and inhibition of the pathway can play a protective role in myocardial ischemiareperfusion injury.
脊髓 / p-ERK / PD98059 / 心肌缺血再灌注损伤 / 大鼠
Spinal cord / p-ERK / PD98059 / Myocardial ischemic-reperfusion injury / Rats
[1]Remme WJ, Kruyssen DA, Look MP, Bootsma M, de Leeuw PW. Systemic and cardiac neuroendocrine activation and severity of myocardial ischemia in humans[J]. J Am Coll Cardiol, 1994, 23(1): 82-91.
[2]Hua F, Ricketts BA, Reifsteck A, Ardell JL, Williams CA. Myocardial ischemia induces the release of substance P from cardiac afferent neurons in rat thoracic spinal cord[J]. Am J Physiol Heart Circ Physiol, 2004, 286(5): H1654-H1664.
[3]Odenstedt J, Linderoth B, Bergfeldt L, Ekre O, Grip L, Mannheimer C, et al. Spinal cord stimulation effects on myocardial ischemia, infarct size, ventricular arrhythmia, and noninvasive electrophysiology in a porcine ischemia-reperfusion model[J]. Heart Rhythm, 2011, 8(6): 892-898.
[4]Bading H. Transcription-dependent neuronal plasticity the nuclear calcium Hypothesis[J]. Eur J Biochem, 2000, 267(17): 5280-5283.
[5]Hardingham GE, Chawla S, Johnson CM, Bading H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression[J]. Nature, 1997, 385(6613): 260-265.
[6]Wiegert JS, Bading H. Activity-dependent calcium signaling and ERK-MAP kinases in neurons: a link to structural plasticity of the nucleus and gene transcription regulation[J]. Cell Calcium, 2011, 49(5): 296-305.
[7]Ji RR, Gereau RW, Malcangio M, Strichartz GR. MAP kinase and pain[J]. Brain Res Rev, 2009, 60(1): 135-148.
[8]Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid Space[J]. Physiol Behav, 1976, 17(6): 1031-1036.
[9]师存伟. 椎管内置入不同型号的导管对大鼠运动感觉功能及脊髓超微结构的影响[J]. 青海医学院学报, 2007, 28(3): 187-191.
[10]Wong GT, Yao L, Xia Z, Irwin MG. Intrathecal morphine remotely preconditions the heart via a neural pathway[J]. J Cardiovasc Pharmacol, 2012, 60(2): 172-178.
[11]杨江帆, 许月珍, 李萍, 杨戎威. 新生儿先天性心脏病患病率的调查和孕妇增补叶酸的预防效果[J]. 中国当代儿科杂志, 2000, 2(5): 320-322,325.
[12]Kaplan JA, Reich DL, Savino JS. Kaplan′s Cardiac Anesthesia: The Echo Era[M]. 6th ed. Missouri: Elsevier Saunders, 2011: 3.
[13]Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy[J]. Am J Cardiol, 2010, 106(3): 360-368.
[14]Kuo DC, Oravitz JJ, DeGroat WC. Tracing of afferent and efferent pathways in the left inferior cardiac nerve of the cat using retrograde and transganglionic transport of horseradish peroxidase[J]. Brain Res, 1984, 321(1): 111-118.
[15]Hua F, Harrison T, Qin C, Reifsteck A, Ricketts B, Carnel C, et al. cFos expression in rat brainstem and spinal cord in response to activation of cardiac ischemia-sensitive afferent neurons and electrostimulatory modulation[J]. Am J Physiol Heart Circ Physiol, 2004, 287(6): H2728-H2738.
[16]O′Donnell A, Odrowaz Z, Sharrocks AD. Immediate-early gene activation by the MAPK pathways: what do and don't we know[J]. Biochem Soc Trans, 2012, 40(1): 58-66.
[17]Ding X, Ardell JL, Hua F, McAuley RJ, Sutherly K, Daniel JJ, et al. Modulation of cardiac ischemia-sensitive afferent neuron signaling by preemptive C2 spinal cord stimulation: effect on substance P release from rat spinal cord[J]. Am J Physiol Regul Integr Comp Physiol, 2008, 294(1): R93-R101.
[18]Hua F, Ricketts BA, Reifsteck A, Ardell JL, Williams CA. Myocardial ischemia induces the release of substance P from cardiac afferent neurons in rat thoracic spinal cord[J]. Am J Physiol Heart Circ Physiol, 2004, 286(5): H1654-H1664.
[19]Ding X, Hua F, Sutherly K, Ardell JL, Williams CA. C2 spinal cord stimulation induces dynorphin release from rat T4 spinal cord: potential modulation of myocardial ischemia-sensitive neurons[J]. Am J Physiol Regul Integr Comp Physiol, 2008, 295(5): R1519-R1528.