 PDF(2606 KB)
PDF(2606 KB)


母孕前和孕期高脂饮食对新生仔鼠骨骼生长的影响及机制探讨
王云峰, 程盼贵, 张知新, 潘琳, 张芳, 刘燕妮, 李鸿
中国当代儿科杂志 ›› 2014, Vol. 16 ›› Issue (11) : 1143-1148.
 PDF(2606 KB)
PDF(2606 KB)
 PDF(2606 KB)
PDF(2606 KB)
母孕前和孕期高脂饮食对新生仔鼠骨骼生长的影响及机制探讨
Effect of maternal high-fat diet before and during pregnancy on bone growth ofneonatal offspring rats
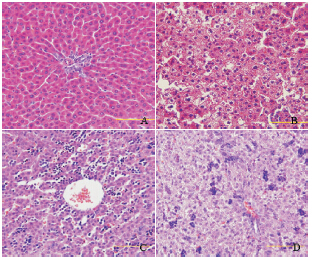
目的 观察母鼠孕前和孕期高脂饮食对新生仔鼠骨骼生长的影响,并探讨影响骨骼生长的机制.方法 40 只雌性Sprague-Dawley 大鼠随机分为高脂组和对照组(n=20),分别喂养35% 高脂饲料和普通饲料.喂养8 周后,高脂组和对照组各取8 只雌鼠观察其肝脏组织病理;其余与普通饲料喂养的雄性大鼠交配,孕期分别继续给予高脂饲料或普通饲料喂养.待娩出新生仔鼠后24 h 内,测量两组仔鼠体长(鼻尾长度);酶联免疫吸附试验测定血清胰岛素样生长因子-I(IGF-I)水平;光镜下观察肝脏组织病理;免疫组织化学法检测长骨(胫骨、股骨)中胰岛素受体底物1(IRS-1)和磷酸化IRS-1(Phospho-IRS-1)的表达;蛋白质印迹技术检测长骨软骨细胞中促分裂原活化蛋白激酶(MAPK)和磷酸化MAPK(Phospho-MAPK)、磷酯酰肌醇3-激酶(PI3K)和磷酸化PI3K(Phospho-PI3K)、蛋白激酶B(AKT1)和磷酸化AKT1(Phospho-AKT1)的蛋白表达.结果 高脂组仔鼠的出生体长较对照组显著降低(P<0.05).高脂组仔鼠血清IGF-I 水平较对照组下降,但差异无统计学意义(P>0.05).高脂组母鼠及仔鼠肝组织可见脂肪样变,而对照组肝脏病理形态正常.两组仔鼠长骨软骨细胞中IRS-1 的表达差异无统计学意义(P>0.05).高脂组仔鼠长骨软骨细胞中MAPK 的表达水平高于对照组(P<0.05),而PI3K 及AKT1/Phospho-AKT1 的表达水平在两组间差异无统计学意义(P>0.05).结论 母鼠孕前和孕期高脂饮食会影响胎鼠在宫内的骨骼发育,可能与IGF-I 的下降有关,但未发现IGF-I 对骨骼影响的确切发病机制.
Objective To explore the mechanism and effect of maternal high-fat diet before and during pregnancyon bone growth of neonatal offspring rats. Methods Forty female Sprague-Dawley rats were divided into high-fatdiet and control groups (n=20) that were fed with 35% high-fat diet and standard chow, respectively. After 8 weeks, 8female rats from each group were sacrificed for liver pathological examinations and the other female rats were matedwith male rats and fed continuously with 35% high-fat diet and standard chow throughout gestation, respectively. Thebody lengths (from apex nasi to end of tail) of the offspring rats from both groups were measured within 24 hours afterbirth. Enzyme-linked immunosorbent assay was used to detect serum insulin-like growth factor (IFG-I) levels. Liverpathological changes were observed under a light microscope. The expression of insulin receptor substrate 1 (IRS-1) and phosphorylation IRS-1 (Phospho-IRS-1) in tibia and femur samples were detected by immunohistochemistry.The expression of mitogen-activated protein kinase (MAPK) and phosphorylation MAPK (Phospho-MAPK),phosphatidylinositol 3-kinase (PI3K) and phosphorylation PI3K (Phospho-PI3K), protein kinase B (AKT1) andphosphorylation AKT1 (Phospho-AKT1) in tibia and femur samples were detected by Western blot. Results Theoffspring rats from the high-fat diet group showed a significant shorter body length compared with those from the control group (P<0.05). The level of serum IGF-I in offspring rats from the high-fat diet group decreased by 20.1% incomparison to those from the control group, but there was no significant difference between the two groups (P>0.05).Fatty degeneration was found in livers of both high-fat diet-fed maternal rats and their offspring rats under a lightmicroscope. There were no significant differences in IRS-1 and Phospho-IRS-1 expression in chondrocytes of tibia andfemur samples between the offspring rats of the two groups (P>0.05). The protein expression of MAPK in chondrocytesof tibia and femur samples of offspring rats from the high-fat diet group was higher than that from the control group(P<0.05). There were no significant differences of PI3K and AKT1/Phospho-AKT1 between the offspring rats of the twogroups (P>0.05). Conclusions A maternal high-fat diet before and during pregnancy may affect the bone growth ofoffspring rats in utero, which is possibly associated with the decreased IGF-I level. However, further study on the exactmechanism of IGF-I on the bone growth is needed.
国家自然科学基金(81270496).