 PDF(2318 KB)
PDF(2318 KB)


沉默Pin1表达抑制高氧诱导人肺泡上皮细胞凋亡
赵帅, 董文斌, 张婵, 李清平, 康兰, 雷小平, 翟雪松
中国当代儿科杂志 ›› 2015, Vol. 17 ›› Issue (5) : 496-501.
 PDF(2318 KB)
PDF(2318 KB)
 PDF(2318 KB)
PDF(2318 KB)
沉默Pin1表达抑制高氧诱导人肺泡上皮细胞凋亡
Silencing of Pin1 suppresses hyperoxia-induced apoptosis of A549 cells
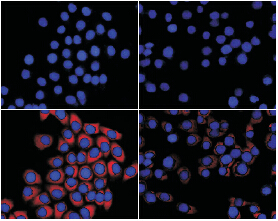
目的 研究沉默Pin1表达对高氧诱导人肺泡上皮细胞(A549细胞)凋亡的影响。方法 在体外常规培养A549细胞, 随机分为对照组、高氧组、空载体组和Pin1-shRNA组, 以3 L/min速度向各处理组细胞中通入95% O2和5% CO2的高纯混合气10 min。培养24 h后, 倒置相差显微镜下观察细胞形态; 流式细胞术检测细胞凋亡率; SP细胞免疫化学法检测细胞凋亡相关蛋白XIAP和Caspase-9表达; 荧光显微镜检测细胞线粒体活性氧簇(ROS)及线粒体膜电位。结果 高氧组和空载体组细胞均生长状态差, Pin1-shRNA组活细胞数量较高氧组和空载体组多, 但比对照组少。与对照组相比, 高氧组和空载体组细胞凋亡率增加、Caspase-9表达增加、XIAP表达降低、ROS含量增加、线粒体膜电位下降(均P<0.05)。而Pin1-shRNA组与高氧组和空载体组相比, 细胞凋亡率下降、Caspase-9表达降低、XIAP表达增加、ROS含量降低、线粒体膜电位升高(均P<0.05), 但与对照组相比差异亦有统计学意义(均P<0.05)。结论 抑制人肺泡上皮细胞Pin1表达可减轻高氧诱导的细胞凋亡。
Objective To explore the effect of silence of Pin1 expression on hyperoxia-induced apoptosis in alveolar epithelial cells A549. Methods A549 cells were divided into four groups: control, hyperoxia, negative lentivirus and Pin1-shRNA hyperoxia. The hyperoxia group was exposed to a mixture of 95%O2 and 5%CO2 for 10 minutes. Then cells were cultured in a closed environment. After 24 hours, the changes of morphology were observed under an inverted microscope. Cell apoptosis was detected by flow cytometry (FCM). The expression of X-linked inhibitor of apoptosis protein (XIAP) and Caspase-9 were detected by immunohistochemistry. The production of reactive oxygen species (ROS) and cellular mitochondria membrane potential (△Ψm) were determined by fluorescence microscopy. Results Under the inverted microscope, the A549 cells grew slowly and the changes in morphology of the cells were most obvious in the hyperoxia and negative lentivirus groups. The changes in morphology of A549 cells were obviously improved in the Pin1-shRNA hyperoxia group. The FCM results showed that the apoptosis rate of A549 cells increased, Caspase-9 expression increased, XIAP expression decreased, mitochondrial ROS production increased and mitochondrial membrane potential decreased in the hyperoxia and negative lentivirus groups compared with the control group (P<0.05). Compared with the hyperoxia and negative lentivirus groups, the apoptosis rate of A549 cells decreased, Caspase-9 expression decreased, XIAP expression increased, mitochondrial ROS production decreased and mitochondrial membrane potential increased in the Pin1-shRNA hyperoxia group (P<0.05), although the levels of the indexes did not reach to those of the control group. Conclusions Silencing of Pin1 could suppress hyperoxia-induced apoptosis of A549 cells.
Pin1 / 高氧 / 氧化应激 / 凋亡 / 人肺泡上皮细胞
Pin1 / Hyperoxia / Oxidative stress / Apoptosis / Alveolar epithelial cell
中华儿科杂志第二届双鹤珂立苏科研基金;四川省教育厅科研基金(08ZA150);四川省卫生厅科研基金(90191);四川省科技厅-泸州市科技局-泸州医学院2014年联合科研项目资金(2014NZ0014);泸州市政府-泸州医学院联合专项资金(2013LZLY-J08)。