 PDF(2648 KB)
PDF(2648 KB)


 PDF(2648 KB)
PDF(2648 KB)
 PDF(2648 KB)
PDF(2648 KB)
胚胎期铅暴露对子代大鼠运动协调能力的影响及可能机制
Effects of embryonic lead exposure on motor function and balance ability in offspring rats and possible mechanisms
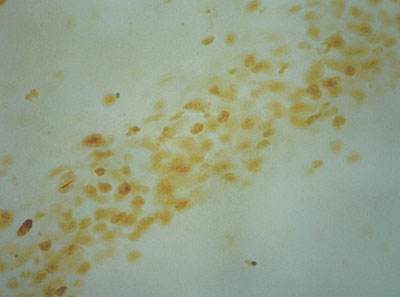
目的 探讨胚胎期铅暴露对子代大鼠运动协调能力的影响及可能机制。方法 采用Sprague-Dawley大鼠孕期自由饮用0.1% (低剂量铅暴露组)和0.2% (高剂量铅暴露组)醋酸铅溶液的方法建立胚胎期铅暴露模型,并设空白对照组。将各组母鼠娩出的雄性仔鼠纳入研究,其中空白对照组12只,低剂量铅暴露组10只,高剂量铅暴露组9只。应用转体实验和衣架实验观察仔鼠运动、协调能力;各组随机取8只大鼠,应用免疫组化、改良Timm's染色法分别观察各组大鼠海马c-Fos蛋白表达及苔藓纤维发芽 (MFS)情况。结果 高剂量铅暴露组转体时间长于正常对照组和低剂量铅暴露组 (P < 0.05),低剂量铅暴露组转体时间亦长于正常对照组 (P < 0.05)。高剂量铅暴露组平衡运动得分低于正常对照组和低剂量铅暴露组 (P < 0.05),低剂量铅暴露组平衡运动得分亦低于正常对照组 (P < 0.05)。高剂量铅暴露组海马CA1区c-Fos阳性细胞面积百分比高于正常对照组和低剂量铅暴露组 (P < 0.05),低剂量铅暴露组c-Fos阳性细胞面积百分比亦高于正常对照组 (P < 0.05)。高剂量铅暴露组CA3区及齿状回MFS的半定量评分均高于正常对照组和低剂量铅暴露组 (P < 0.05);低剂量铅暴露组CA3区及齿状回MFS的半定量评分亦高于正常对照组 (P < 0.05)。结论 胚胎期铅暴露损害子代大鼠运动协调能力,该变化可能与海马CA1区c-Fos蛋白表达增加及海马CA3区、齿状回发生异常MFS有关。
Objective To explore the effects of embryonic lead exposure on motor function and balance ability in offspring rats and the possible mechanisms. Methods An animal model of embryonic lead exposure was prepared with the use of pregnant Sprague-Dawley rats freely drinking 0.1% (low-dose group, LG) or 0.2% (high-dose group, HG) lead acetate solution. A normal control group (NG) was also set. The male offspring rats of these pregnant rats were included in the study, consisting of 12 rats in the NG group, 10 rats in the LG group, and 9 rats in the HG group. The offspring rats' motor function and balance ability were evaluated using body turning test and coat hanger test. Eight rats were randomly selected from each group, and immunohistochemistry and Timm's staining were employed to measure the expression of c-Fos and mossy fiber sprouting (MFS) in the hippocampus. Results The HG group had a significantly longer body turning time than the NG and LG groups (P < 0.05), and the LG group had a significantly longer body turning time than the NG group (P < 0.05). The HG group had a significantly lower score of balance ability than the NG and LG groups (P < 0.05), and the LG group had a significantly lower score of balance ability than the NG group (P < 0.05). The area percentage of c-Fos-positive neurons in the hippocampal CA1 region was significantly higher in the HG group than in the other two groups (P < 0.05), and it was significantly higher in the LG group than in the NG group (P < 0.05). The semi-quantitative scores of MFS in the hippocampal CA3 region and dentate gyrus were significantly higher in the HG group than in the other two groups (P < 0.05), and they were significantly higher in the LG group than in the NG group (P < 0.05). Conclusions Embryonic lead exposure could impair the offspring rats' motor function and balance ability. These changes may be related to increased c-Fos expression in the hippocampal CA3 region and abnormal MFS in the hippocampal CA3 region and dentate gyrus.
运动功能 / 平衡能力 / 精神发育迟滞 / 胚胎期 / 铅暴露 / 大鼠
Motor function / Balance ability / Mental retardation / Embryonic period / Lead exposure / Rats
[1] 林国祯, 吴家刚, 彭荣飞, 等. 广州市使用无铅汽油十年后儿童血铅水平调查[J]. 中华预防医学杂志, 2008, 42(10):727-730.
[2] van der Kuijp TJ, Huang L, Cherry CR. Health hazards of China's lead-acid battery industry:a review of its market drivers, production processes, and health impacts[J]. Environ Health, 2013, 12:61.
[3] Mazumdar I, Goswami K. Congenital lead poisoning:an unusual presentation[J]. Indian J Clin Biochem, 2014, 29(2):257-259.
[4] Nevin R. Trends in preschool lead exposure, mental retardation, and scholastic achievement:association or causation?[J]. Environ Res, 2009, 109(3):301-310.
[5] 张艳淑, 郝玉兰, 闫立成, 等. 槲皮素对铅致大鼠某些神经功能损伤缓解作用研究[J]. 中国职业医学, 2011, 38(4):302-305.
[6] 张琳, 邹时朴, 李国林. 高血铅对幼儿智力发育的影响[J]. 南昌大学学报(医学版), 2014, 54(6):65-66, 69.
[7] 孙慧生. 铅中毒儿童运动功能与心理学特点及干预措施的临床研究[J]. 中国健康心理学杂志, 2006, 14(1):102-104.
[8] Alba A, Carleton L, Dinkel L, et al. Increased lead levels in pregnancy among immigrant women[J]. J Midwifery Womens Health, 2012, 57(5):509-514.
[9] Galván EJ, Cosgrove KE, Barrionuevo G. Multiple forms of long-term synaptic plasticity at hippocampal mossy fiber synapses on interneurons[J]. Neuropharmacology, 2011, 60(5):740-747.
[10] Toscano-Silva M, Gomes da Silva S, Scorza FA, et al. Hippocampal mossy fiber sprouting induced by forced and voluntary physical exercise[J]. Physiol Behav, 2010, 101(2):302-308.
[11] Siddiqui AH, Joseph SA. CA3 axonal sprouting in kainite-induced chronic epilepsy[J]. Brain Res, 2005, 1066(1-2):129-146.
[12] 周戬平, 王帆, 黄剑锋, 等. 孕期铅暴露对子代大鼠海马区神经元超微结构及突触形态学影响的研究[J]. 中国儿童保健杂志, 2012, 20(3):231-234.
[13] 周戬平, 王帆, 杨玉凤, 等. 孕期铅暴露对子代大鼠情感行为及学习记忆变化的实验研究[J]. 中国儿童保健杂志, 2012, 20(2):135-138.
[14] Jazayeri SB, Firouzi M, Abdollah Zadegan S, et al. The effect of timing of decompression on neurologic recovery and histopathologic findings after spinal cord compression in a rat model[J]. Acta Med Iran, 2013, 51(7):431-437.
[15] Vijayaprakash KM, Sridharan N. An experimental spinal cord injury rat model using customized impact device:a cost-effective approach[J]. J Pharmacol Pharmacother, 2013, 4(3):211-213.
[16] 宋青, 孟书聪, 董晓敏, 等. 改良的Timm染色法显示大鼠海马苔藓纤维末梢出芽的变化[J]. 四川解剖学杂志, 2001, 9(2):98.
[17] 马勋泰, 晏勇, 王学峰. 皮质发育障碍大鼠小脑回形态学和海马苔藓纤维发芽的研究[J].中华神经医学杂志, 2005, 4(4):334-337.
[18] 孙晓彩, 李力, 张敏, 等. Wistar大鼠海马CA1区、CA3区和齿状回区的解剖分割[J].中国应用生理学杂志, 2012, 28(2):189-192.
[19] Lui MM, Lam DC, Ip MS. Significance of endothelial dysfunction in sleep-related breathing disorder[J]. Respirology, 2013, 18(1):39-46.
[20] 吴超, 林孟群, 林贤文. 颅脑损伤与c-Fos基因的研究进展[J]. 四川生理科学杂志, 2011, 33(1):34-36.
[21] 蒋犁, 丁艳洁, 晋光荣. 缺血缺氧后新生鼠脑c-fos表达与脑海马迟发性神经元死亡[J].中华围产医学杂志, 2001, 4:247-250.
[22] Park JK, Lee SJ, Oh CS. Treadmill exercise exerts ameliorating effect on isolation-induced depression via neuronal activation[J]. J Exerc Rehabil, 2013, 9(2):234-242.
[23] 陈衍晨, 赵丹, 卿娣, 等. 缺氧再灌注斑马鱼胚胎脑部细胞凋亡及c-fos基因的表达[J]. 中国组织工程研究, 2013, 17(37):6613-6619.
[24] Shizuki K, Ogawa K, Matsunobu T, et al. Expression of c-Fos after noise-induced temporary threshold shift in the guinea pig cochlea[J]. Neurosci Lett, 2002, 320(1-2):73-76.
[25] Hunt RF, Haselhorst LA, Schoch KM, et al. Posttraumatic mossy fiber sprouting is related to the degree of cortical damage in three mouse strains[J]. Epilepsy Res, 2012, 99(1-2):167-170.
[26] Safiulina VF, Fattorini G, Conti F, et al. GABAergic signaling at mossy fiber synapses in neonatal rat hippocampus[J]. J Neurosci, 2006, 26(2):597-608.
[27] Lobo MK, Itri JN, Cepeda C, et al. Ionotropic glutamate receptor expression and dopaminergic modulation in the developing subthalamic nucleus of the rat:an immunohistochemical and electrophysiological analysis[J]. Dev Neurosci, 2003, 25(6):384-393.
[28] Jeffery KJ, Hayman R. Plasticity of the hippocampal place cell representation[J]. Rev Neurosci, 2004, 15(5):309-331.
[29] Gomes FG, Gomes Da Silva S, Cavalheiro EA, et al. Beneficial influence of physical exercise following status epilepticus in the immature brain of rats[J]. Neuroscience, 2014, 274:69-81.
[30] Ni H, Li C, Tao LY, et al. Physical exercise improves learning by modulating hippocampal mossy fiber sprouting and related gene expression in a developmental rat model of penicillin-induced recurrent epilepticus[J]. Toxicol Lett, 2009, 191(1):26-32.
陕西省科技攻关项目(2016SF-263);陕西省人民医院孵化基金(2010)。