 PDF(2157 KB)
PDF(2157 KB)


Relationship between expression of endothelial nitric oxide synthase and NADPH oxidase in lungs of mice exposed to chronic hypoxia
WU Xi-Ling, DU Li-Zhong, XU Xue-Feng
Chinese Journal of Contemporary Pediatrics ›› 2015, Vol. 17 ›› Issue (9) : 1001-1006.
 PDF(2157 KB)
PDF(2157 KB)
 PDF(2157 KB)
PDF(2157 KB)
Relationship between expression of endothelial nitric oxide synthase and NADPH oxidase in lungs of mice exposed to chronic hypoxia
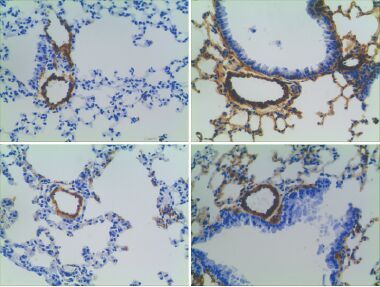
Objective To explore the relationship between the expression of endothelial nitric oxide synthase (eNOS) and NADPH oxidase (NOX) in the lungs of mice treated by chronic hypoxic exposure.Methods Thirty male wild-type (WT) C57Bl/6 mice and thirty male eNOS-knockout (KO) C57BL/6 mice were randomly divided into normoxic groups (exposed to normoxia for 7 days or 21 days), hypoxic groups (exposed to 10% oxygen for 7 days or 21 days), and treatment groups (exposed to 10% oxygen and orally administrated 10 mmol/L 4-hydroxy TEMPO in drinking water for 7 days or 21 days) (n=6 in each group). The remodeling of the small pulmonary arteries was evaluated by the percentage of media wall thickness (MT%). The weight ratio of right ventricle to left ventricle plus septum (RV/[LV+S]) was calculated to evaluate the hypertrophy of right ventricle. Real-time PCR was used to measure the mRNA expression of NOX2, NOX4, and eNOS in mouse lungs. ELISA was used to determine the concentration of reactive oxygen species (ROS) in mouse lungs.Results In WT mice and KO mice, the hypoxic groups had significantly increased pulmonary vascular remodeling and RV/[LV+S] compared with the normoxic and treatment groups (P<0.05), but there were no significant differences between the normoxic and treatment groups (P>0.05). In WT mice, the hypoxic and treatment groups had significantly lower ROS concentrations than the normoxic group (P<0.05), but there were no significant differences between the hypoxic and treatment groups (P>0.05). In WT mice, the mRNA expression of eNOS, NOX2, and NOX4 was significantly higher in the hypoxic group than in the normoxic group (P<0.05), and 4-hydroxy TEMPO reversed their over-expression. In the normoxic group, the KO mice had significantly higher NOX2 and NOX4 mRNA expression than the WT mice (P<0.05); in KO mice, the hypoxic group showed no significant changes in NOX4 mRNA expression (P>0.05), but had significantly reduced NOX2 mRNA expression (P<0.05), as compared with the normoxic group; the treatment group had reduced expression of NOX2 mRNA expression and increased NOX4 mRNA expression (P<0.05), as compared with the hypoxic group.Conclusions eNOS plays a key role in the regulation of expression of NOX2 and NOX4 in the lungs exposed to hypoxia. It suggests that NOX and eNOS may physically interact with one another in pulmonary vascular remodeling induced by chronic hypoxia.
Endothelial nitric oxide synthase / NADPH oxidase 2 / NADPH oxidase 4 / 4-hydroxy TEMPO / Reactive oxygen species / Mice